Positively charged bioactive Ti metal prepared by simple chemical and heat treatments
- PMID: 20444711
- PMCID: PMC3024574
- DOI: 10.1098/rsif.2010.0129.focus
Positively charged bioactive Ti metal prepared by simple chemical and heat treatments
Abstract
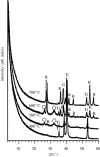
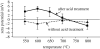
A highly bioactive bone-bonding Ti metal was obtained when Ti metal was simply heat-treated after a common acid treatment. This bone-bonding property was ascribed to the formation of apatite on the Ti metal in a body environment. The formation of apatite on the Ti metal was induced neither by its surface roughness nor by the rutile phase precipitated on its surface, but by its positively charged surface. The surface of the Ti metal was positively charged because acid groups were adsorbed on titanium hydride formed on the Ti metal by the acid treatment, and remained even after the titanium hydride was transformed into titanium oxide by the subsequent heat treatment. These results provide a new principle based on a positively charged surface for obtaining bioactive materials.
Figures


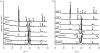





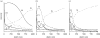

Similar articles
-
Bone-bonding properties of Ti metal subjected to acid and heat treatments.J Mater Sci Mater Med. 2012 Dec;23(12):2981-92. doi: 10.1007/s10856-012-4758-4. Epub 2012 Sep 5. J Mater Sci Mater Med. 2012. PMID: 22948713
-
Effect of heat treatments on apatite-forming ability of NaOH- and HCl-treated titanium metal.J Mater Sci Mater Med. 2011 Feb;22(2):273-8. doi: 10.1007/s10856-010-4218-y. Epub 2010 Dec 29. J Mater Sci Mater Med. 2011. PMID: 21188481
-
Effect of HCl concentrations on apatite-forming ability of NaOH-HCl- and heat-treated titanium metal.J Mater Sci Mater Med. 2009 Dec;20(12):2401-11. doi: 10.1007/s10856-009-3815-0. J Mater Sci Mater Med. 2009. PMID: 19585225
-
Novel bioactive materials developed by simulated body fluid evaluation: Surface-modified Ti metal and its alloys.Acta Biomater. 2016 Oct 15;44:16-30. doi: 10.1016/j.actbio.2016.08.013. Epub 2016 Aug 10. Acta Biomater. 2016. PMID: 27521496 Review.
-
Bioactive metals: preparation and properties.J Mater Sci Mater Med. 2004 Feb;15(2):99-107. doi: 10.1023/b:jmsm.0000011809.36275.0c. J Mater Sci Mater Med. 2004. PMID: 15330042 Review.
Cited by
-
Bone-bonding properties of Ti metal subjected to acid and heat treatments.J Mater Sci Mater Med. 2012 Dec;23(12):2981-92. doi: 10.1007/s10856-012-4758-4. Epub 2012 Sep 5. J Mater Sci Mater Med. 2012. PMID: 22948713
-
Growth of Novel Ceramic Layers on Metals via Chemical and Heat Treatments for Inducing Various Biological Functions.Front Bioeng Biotechnol. 2015 Oct 27;3:176. doi: 10.3389/fbioe.2015.00176. eCollection 2015. Front Bioeng Biotechnol. 2015. PMID: 26579517 Free PMC article. Review.
-
Improvement of hydroxyapatite formation ability of titanium-based alloys by combination of acid etching and apatite nuclei precipitation.IET Nanobiotechnol. 2020 Oct;14(8):688-694. doi: 10.1049/iet-nbt.2020.0053. IET Nanobiotechnol. 2020. PMID: 33108325 Free PMC article.
-
Effects of surface charges on dental implants: past, present, and future.Int J Biomater. 2012;2012:381535. doi: 10.1155/2012/381535. Epub 2012 Oct 8. Int J Biomater. 2012. PMID: 23093962 Free PMC article.
-
Recent updates for biomaterials used in total hip arthroplasty.Biomater Res. 2018 Dec 5;22:33. doi: 10.1186/s40824-018-0144-8. eCollection 2018. Biomater Res. 2018. PMID: 30534414 Free PMC article. Review.
References
-
- Gold J. M., Schmidt M., Steinemann S. G. 1989. XPS study of amino-acid adsorption to titanium surface. Helv. Phys. Acta 62, 246–249.
-
- Kawanabe K., Ise K., Goto K., Akiyama H., Nakamura T., Kaneuji A., Sugimori T., Matsumoto T. 2009. A new cementless total hip arthoplasty with bioactive titanium porous-coating by alkaline and heat treatment: average 4.8-year results. J. Biomed. Mater. Res. 90B, 476–481. (10.1002/jbm.b.31309) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

