Hypoxic regulation of erythropoiesis and iron metabolism
- PMID: 20444740
- PMCID: PMC2904169
- DOI: 10.1152/ajprenal.00174.2010
Hypoxic regulation of erythropoiesis and iron metabolism
Abstract
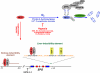
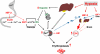
The kidney is a highly sensitive oxygen sensor and plays a central role in mediating the hypoxic induction of red blood cell production. Efforts to understand the molecular basis of oxygen-regulated erythropoiesis have led to the identification of erythropoietin (EPO), which is essential for normal erythropoiesis and to the purification of hypoxia-inducible factor (HIF), the transcription factor that regulates EPO synthesis and mediates cellular adaptation to hypoxia. Recent insights into the molecular mechanisms that control and integrate cellular and systemic erythropoiesis-promoting hypoxia responses and their potential as a therapeutic target for the treatment of renal anemia are discussed in this review.
Figures
References
-
- An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature 392: 405–408, 1998 - PubMed
-
- Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Liu E, Sergueeva AI, Miasnikova GY, Mole D, Maxwell PH, Stockton DW, Semenza GL, Prchal JT. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet 32: 614–621, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

