EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs
- PMID: 20444750
- PMCID: PMC2935329
- DOI: 10.1136/ard.2009.126532
EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs
Erratum in
- Ann Rheum Dis. 2011 Aug;70(8):1519
Abstract
Treatment of rheumatoid arthritis (RA) may differ among rheumatologists and currently, clear and consensual international recommendations on RA treatment are not available. In this paper recommendations for the treatment of RA with synthetic and biological disease-modifying antirheumatic drugs (DMARDs) and glucocorticoids (GCs) that also account for strategic algorithms and deal with economic aspects, are described. The recommendations are based on evidence from five systematic literature reviews (SLRs) performed for synthetic DMARDs, biological DMARDs, GCs, treatment strategies and economic issues. The SLR-derived evidence was discussed and summarised as an expert opinion in the course of a Delphi-like process. Levels of evidence, strength of recommendations and levels of agreement were derived. Fifteen recommendations were developed covering an area from general aspects such as remission/low disease activity as treatment aim via the preference for methotrexate monotherapy with or without GCs vis-à-vis combination of synthetic DMARDs to the use of biological agents mainly in patients for whom synthetic DMARDs and tumour necrosis factor inhibitors had failed. Cost effectiveness of the treatments was additionally examined. These recommendations are intended to inform rheumatologists, patients and other stakeholders about a European consensus on the management of RA with DMARDs and GCs as well as strategies to reach optimal outcomes of RA, based on evidence and expert opinion.
Conflict of interest statement
Figures
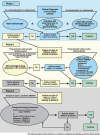
References
-
- Smolen JS, Aletaha D, Koeller M, et al. New therapies for treatment of rheumatoid arthritis. Lancet 2007;370:1861–74 - PubMed
-
- Svensson B, Boonen A, Albertsson K, et al. Low-dose prednisolone in addition to the initial disease-modifying antirheumatic drug in patients with early active rheumatoid arthritis reduces joint destruction and increases the remission rate: a two-year randomized trial. Arthritis Rheum 2005;52:3360–70 - PubMed
-
- Wassenberg S, Rau R, Steinfeld P, et al. Very low-dose prednisolone in early rheumatoid arthritis retards radiographic progression over two years: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 2005;52:3371–80 - PubMed
-
- van Everdingen AA, Jacobs JW, Siewertsz van Reesema DR, et al. Low-dose prednisone therapy for patients with early active rheumatoid arthritis: clinical efficacy, disease-modifying properties, and side effects: a randomized, double-blind, placebo-controlled clinical trial. Ann Intern Med 2002;136:1–12 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

