HotPoint: hot spot prediction server for protein interfaces
- PMID: 20444871
- PMCID: PMC2896123
- DOI: 10.1093/nar/gkq323
HotPoint: hot spot prediction server for protein interfaces
Abstract
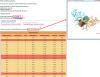
The energy distribution along the protein-protein interface is not homogenous; certain residues contribute more to the binding free energy, called 'hot spots'. Here, we present a web server, HotPoint, which predicts hot spots in protein interfaces using an empirical model. The empirical model incorporates a few simple rules consisting of occlusion from solvent and total knowledge-based pair potentials of residues. The prediction model is computationally efficient and achieves high accuracy of 70%. The input to the HotPoint server is a protein complex and two chain identifiers that form an interface. The server provides the hot spot prediction results, a table of residue properties and an interactive 3D visualization of the complex with hot spots highlighted. Results are also downloadable as text files. This web server can be used for analysis of any protein-protein interface which can be utilized by researchers working on binding sites characterization and rational design of small molecules for protein interactions. HotPoint is accessible at http://prism.ccbb.ku.edu.tr/hotpoint.
Figures

References
-
- Bogan AA, Thorn KS. Anatomy of hot spots in protein interfaces. J. Mol. Biol. 1998;280:1–9. - PubMed
-
- Clackson T, Wells JA. A hot spot of binding energy in a hormone-receptor interface. Science. 1995;267:383–386. - PubMed
-
- Wells JA. Systematic mutational analyses of protein-protein interfaces. Meth. Enzymol. 1991;202:390–411. - PubMed
-
- Keskin O, Ma B, Nussinov R. Hot regions in protein–protein interactions: the organization and contribution of structurally conserved hot spot residues. J. Mol. Biol. 2005;345:1281–1294. - PubMed
-
- Keskin O, Gursoy A, Ma B, Nussinov R. Principles of protein–protein interactions: what are the preferred ways for proteins to interact? Chem. Rev. 2008;108:1225–1244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

