Adenovirus late-phase infection is controlled by a novel L4 promoter
- PMID: 20444889
- PMCID: PMC2898241
- DOI: 10.1128/JVI.00107-10
Adenovirus late-phase infection is controlled by a novel L4 promoter
Abstract
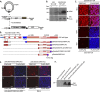
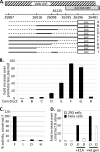
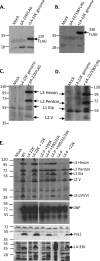
During human adenovirus 5 infection, a temporal cascade of gene expression leads ultimately to the production of large amounts of the proteins needed to construct progeny virions. However, the mechanism for the activation of the major late gene that encodes these viral structural proteins has not been well understood. We show here that two key positive regulators of the major late gene, L4-22K and L4-33K, previously thought to be expressed under the control of the major late promoter itself, initially are expressed from a novel promoter that is embedded within the major late gene and dedicated to their expression. This L4 promoter is required for late gene expression and is activated by a combination of viral protein activators produced during the infection, including E1A, E4 Orf3, and the intermediate-phase protein IVa2, and also by viral genome replication. This new understanding redraws the long-established view of how adenoviral gene expression patterns are controlled and offers new ways to manipulate that gene expression cascade for adenovirus vector applications.
Figures

References
-
- Akusjärvi, G., and H. Persson. 1981. Controls of RNA splicing and termination in the major late adenovirus transcription unit. Nature 292:420-426. - PubMed
-
- Baker, C. C., and E. B. Ziff. 1981. Promoters and heterogeneous 5′ termini of the messenger-RNAs of adenovirus serotype-2. J. Mol. Biol. 149:189-221. - PubMed
-
- Berk, A. J. 2006. Adenoviridae: the viruses and their replication, p. 63. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, and M. A. Martin (ed.), Fields virology, 5th ed., vol. 2. Lippincott Williams and Wilkins, New York, NY.
-
- Berk, A. J., F. Lee, T. Harrison, J. Williams, and P. A. Sharp. 1979. Pre-early adenovirus-5 gene-product regulates synthesis of early viral messenger-RNAs. Cell 17:935-944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

