Inefficient control of host gene expression by the 2009 pandemic H1N1 influenza A virus NS1 protein
- PMID: 20444891
- PMCID: PMC2898253
- DOI: 10.1128/JVI.00081-10
Inefficient control of host gene expression by the 2009 pandemic H1N1 influenza A virus NS1 protein
Abstract
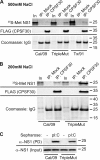
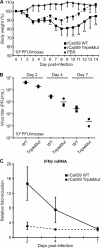
In 2009, a novel swine-origin H1N1 influenza A virus emerged. Here, we characterize the multifunctional NS1 protein of this human pandemic virus in order to understand factors that may contribute to replication efficiency or pathogenicity. Although the 2009 H1N1 virus NS1 protein (2009/NS1) is an effective interferon antagonist, we found that this NS1 (unlike those of previous human-adapted influenza A viruses) is unable to block general host gene expression in human or swine cells. This property could be restored in 2009/NS1 by replacing R108, E125, and G189 with residues corresponding to human virus consensus. Mechanistically, these previously undescribed mutations acted by increasing binding of 2009/NS1 to the cellular pre-mRNA processing protein CPSF30. A recombinant 2009 H1N1 influenza A virus (A/California/04/09) expressing NS1 with these gain-of-function substitutions was more efficient than the wild type at antagonizing host innate immune responses in primary human epithelial cells. However, such mutations had no significant effect on virus replication in either human or swine tissue culture substrates. Surprisingly, in a mouse model of pathogenicity, the mutant virus appeared to cause less morbidity, and was cleared faster, than the wild type. The mutant virus also demonstrated reduced titers in the upper respiratory tracts of ferrets; however, contact and aerosol transmissibility of the virus was unaffected. Our data highlight a potential human adaptation of NS1 that seems absent in "classically derived" swine-origin influenza A viruses, including the 2009 H1N1 virus. We discuss the impact that a natural future gain of this NS1 function may have on the new pandemic virus in humans.
Figures

References
-
- Anonymous. Swine influenza A (H1N1) infection in two children—Southern California, March-April 2009. MMWR Morb. Mortal. Wkly. Rep. 58:400-402. - PubMed
-
- Basler, C. F., A. H. Reid, J. K. Dybing, T. A. Janczewski, T. G. Fanning, H. Zheng, M. Salvatore, M. L. Perdue, D. E. Swayne, A. Garcia-Sastre, P. Palese, and J. K. Taubenberger. 2001. Sequence of the 1918 pandemic influenza virus nonstructural gene (NS) segment and characterization of recombinant viruses bearing the 1918 NS genes. Proc. Natl. Acad. Sci. U. S. A. 98:2746-2751. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

