Ebola virus glycoprotein counteracts BST-2/Tetherin restriction in a sequence-independent manner that does not require tetherin surface removal
- PMID: 20444895
- PMCID: PMC2898217
- DOI: 10.1128/JVI.02636-09
Ebola virus glycoprotein counteracts BST-2/Tetherin restriction in a sequence-independent manner that does not require tetherin surface removal
Abstract
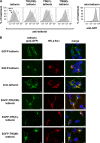
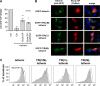
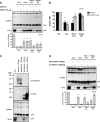
BST-2/tetherin is an interferon-inducible protein that restricts the release of enveloped viruses from the surface of infected cells by physically linking viral and cellular membranes. It is present at both the cell surface and in a perinuclear region, and viral anti-tetherin factors including HIV-1 Vpu and HIV-2 Env have been shown to decrease the cell surface population. To map the domains of human tetherin necessary for both virus restriction and sensitivity to viral anti-tetherin factors, we constructed a series of tetherin derivatives and assayed their activity. We found that the cytoplasmic tail (CT) and transmembrane (TM) domains of tetherin alone produced its characteristic cellular distribution, while the ectodomain of the protein, which includes a glycosylphosphatidylinositol (GPI) anchor, was sufficient to restrict virus release when presented by the CT/TM regions of a different type II membrane protein. To counteract tetherin restriction and remove it from the cell surface, HIV-1 Vpu required the specific sequence present in the TM domain of human tetherin. In contrast, the HIV-2 Env required only the ectodomain of the protein and was sensitive to a point mutation in this region. Strikingly, the anti-tetherin factor, Ebola virus GP, was able to overcome restriction conferred by both tetherin and a series of functional tetherin derivatives, including a wholly artificial tetherin molecule. Moreover, GP overcame restriction without significantly removing tetherin from the cell surface. These findings suggest that Ebola virus GP uses a novel mechanism to circumvent tetherin restriction.
Figures
Similar articles
-
Anti-tetherin activities of HIV-1 Vpu and Ebola virus glycoprotein do not involve removal of tetherin from lipid rafts.J Virol. 2012 May;86(10):5467-80. doi: 10.1128/JVI.06280-11. Epub 2012 Mar 7. J Virol. 2012. PMID: 22398279 Free PMC article.
-
Vpu downmodulates two distinct targets, tetherin and gibbon ape leukemia virus envelope, through shared features in the Vpu cytoplasmic tail.PLoS One. 2012;7(12):e51741. doi: 10.1371/journal.pone.0051741. Epub 2012 Dec 19. PLoS One. 2012. PMID: 23284757 Free PMC article.
-
Simian immunodeficiency virus envelope glycoprotein counteracts tetherin/BST-2/CD317 by intracellular sequestration.Proc Natl Acad Sci U S A. 2009 Dec 8;106(49):20889-94. doi: 10.1073/pnas.0907075106. Epub 2009 Oct 28. Proc Natl Acad Sci U S A. 2009. PMID: 19864625 Free PMC article.
-
Antiviral activity of the interferon-induced cellular protein BST-2/tetherin.AIDS Res Hum Retroviruses. 2009 Dec;25(12):1197-210. doi: 10.1089/aid.2009.0253. AIDS Res Hum Retroviruses. 2009. PMID: 19929170 Free PMC article. Review.
-
Transmembrane interactions of HIV-1 Vpu and tetherin.Curr HIV Res. 2012 Jun;10(4):292-7. doi: 10.2174/157016212800792450. Curr HIV Res. 2012. PMID: 22524177 Review.
Cited by
-
How Ebola virus counters the interferon system.Zoonoses Public Health. 2012 Sep;59 Suppl 2(Suppl 2):116-31. doi: 10.1111/j.1863-2378.2012.01454.x. Zoonoses Public Health. 2012. PMID: 22958256 Free PMC article. Review.
-
Structural Basis for the Antiviral Activity of BST-2/Tetherin and Its Viral Antagonism.Front Microbiol. 2011 Dec 12;2:250. doi: 10.3389/fmicb.2011.00250. eCollection 2011. Front Microbiol. 2011. PMID: 22180752 Free PMC article.
-
HIV-1 Vpu targets cell surface markers CD4 and BST-2 through distinct mechanisms.Mol Aspects Med. 2010 Oct;31(5):407-17. doi: 10.1016/j.mam.2010.08.002. Epub 2010 Sep 19. Mol Aspects Med. 2010. PMID: 20858517 Free PMC article. Review.
-
Influenza A virus does not encode a tetherin antagonist with Vpu-like activity and induces IFN-dependent tetherin expression in infected cells.PLoS One. 2012;7(8):e43337. doi: 10.1371/journal.pone.0043337. Epub 2012 Aug 27. PLoS One. 2012. PMID: 22952667 Free PMC article.
-
Reactomes of porcine alveolar macrophages infected with porcine reproductive and respiratory syndrome virus.PLoS One. 2013;8(3):e59229. doi: 10.1371/journal.pone.0059229. Epub 2013 Mar 19. PLoS One. 2013. PMID: 23527143 Free PMC article.
References
-
- Bour, S., H. Akari, E. Miyagi, and K. Strebel. 2003. Naturally occurring amino acid substitutions in the HIV-2 ROD envelope glycoprotein regulate its ability to augment viral particle release. Virology 309:85-98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

