Vif of feline immunodeficiency virus from domestic cats protects against APOBEC3 restriction factors from many felids
- PMID: 20444897
- PMCID: PMC2898260
- DOI: 10.1128/JVI.00209-10
Vif of feline immunodeficiency virus from domestic cats protects against APOBEC3 restriction factors from many felids
Abstract
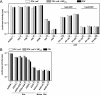
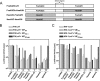
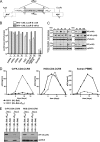
To get more insight into the role of APOBEC3 (A3) cytidine deaminases in the species-specific restriction of feline immunodeficiency virus (FIV) of the domestic cat, we tested the A3 proteins present in big cats (puma, lion, tiger, and lynx). These A3 proteins were analyzed for expression and sensitivity to the Vif protein of FIV. While A3Z3s and A3Z2-Z3s inhibited Deltavif FIV, felid A3Z2s did not show any antiviral activity against Deltavif FIV or wild-type (wt) FIV. All felid A3Z3s and A3Z2-Z3s were sensitive to Vif of the domestic cat FIV. Vif also induced depletion of felid A3Z2s. Tiger A3s showed a moderate degree of resistance against the Vif-mediated counter defense. These findings may imply that the A3 restriction system does not play a major role to prevent domestic cat FIV transmission to other Felidae. In contrast to the sensitive felid A3s, many nonfelid A3s actively restricted wt FIV replication. To test whether Vif(FIV) can protect also the distantly related human immunodeficiency virus type 1 (HIV-1), a chimeric HIV-1.Vif(FIV) was constructed. This HIV-1.Vif(FIV) was replication competent in nonpermissive feline cells expressing human CD4/CCR5 that did not support the replication of wt HIV-1. We conclude that the replication of HIV-1 in some feline cells is inhibited only by feline A3 restriction factors and the absence of the appropriate receptor or coreceptor.
Figures



Similar articles
-
Expression of APOBEC3 Lentiviral Restriction Factors in Cats.Viruses. 2019 Sep 7;11(9):831. doi: 10.3390/v11090831. Viruses. 2019. PMID: 31500260 Free PMC article.
-
Determinants of FIV and HIV Vif sensitivity of feline APOBEC3 restriction factors.Retrovirology. 2016 Jul 1;13(1):46. doi: 10.1186/s12977-016-0274-9. Retrovirology. 2016. PMID: 27368163 Free PMC article.
-
Feline Immunodeficiency Virus Vif N-Terminal Residues Selectively Counteract Feline APOBEC3s.J Virol. 2016 Nov 14;90(23):10545-10557. doi: 10.1128/JVI.01593-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27630243 Free PMC article.
-
Feline APOBEC3s, Barriers to Cross-Species Transmission of FIV?Viruses. 2018 Apr 10;10(4):186. doi: 10.3390/v10040186. Viruses. 2018. PMID: 29642583 Free PMC article. Review.
-
Evolution of feline immunodeficiency virus in Felidae: implications for human health and wildlife ecology.Vet Immunol Immunopathol. 2008 May 15;123(1-2):32-44. doi: 10.1016/j.vetimm.2008.01.010. Epub 2008 Jan 19. Vet Immunol Immunopathol. 2008. PMID: 18359092 Free PMC article. Review.
Cited by
-
A Naturally Occurring Domestic Cat APOBEC3 Variant Confers Resistance to Feline Immunodeficiency Virus Infection.J Virol. 2015 Oct 21;90(1):474-85. doi: 10.1128/JVI.02612-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26491161 Free PMC article.
-
Productive replication and evolution of HIV-1 in ferret cells.J Virol. 2012 Feb;86(4):2312-22. doi: 10.1128/JVI.06035-11. Epub 2011 Dec 14. J Virol. 2012. PMID: 22171279 Free PMC article.
-
Expression of APOBEC3 Lentiviral Restriction Factors in Cats.Viruses. 2019 Sep 7;11(9):831. doi: 10.3390/v11090831. Viruses. 2019. PMID: 31500260 Free PMC article.
-
New World feline APOBEC3 potently controls inter-genus lentiviral transmission.Retrovirology. 2018 Apr 10;15(1):31. doi: 10.1186/s12977-018-0414-5. Retrovirology. 2018. PMID: 29636069 Free PMC article.
-
Evolution of puma lentivirus in bobcats (Lynx rufus) and mountain lions (Puma concolor) in North America.J Virol. 2014 Jul;88(14):7727-37. doi: 10.1128/JVI.00473-14. Epub 2014 Apr 16. J Virol. 2014. PMID: 24741092 Free PMC article.
References
-
- An, P., G. Bleiber, P. Duggal, G. Nelson, M. May, B. Mangeat, I. Alobwede, D. Trono, D. Vlahov, S. Donfield, J. J. Goedert, J. Phair, S. Buchbinder, S. J. O'Brien, A. Telenti, and C. A. Winkler. 2004. APOBEC3G genetic variants and their influence on the progression to AIDS. J. Virol. 78:11070-11076. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

