Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques
- PMID: 20444898
- PMCID: PMC2898229
- DOI: 10.1128/JVI.00410-10
Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques
Abstract
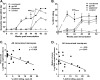
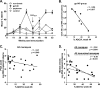
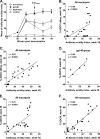
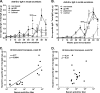
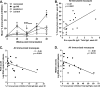
We have shown that following priming with replicating adenovirus type 5 host range mutant (Ad5hr)-human immunodeficiency virus (HIV)/simian immunodeficiency virus (SIV) recombinants, boosting with gp140 envelope protein enhances acute-phase protection against intravenous simian/human immunodeficiency virus (SHIV)(89.6P) challenge compared to results with priming and no boosting or boosting with an HIV polypeptide representing the CD4 binding site of gp120. We retrospectively analyzed antibodies in sera and rectal secretions from these same macaques, investigating the hypothesis that vaccine-elicited nonneutralizing antibodies contributed to the better protection. Compared to other immunized groups or controls, the gp140-boosted group exhibited significantly greater antibody activities mediating antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cell-mediated viral inhibition (ADCVI) in sera and transcytosis inhibition in rectal secretions. ADCC and ADCVI activities were directly correlated with antibody avidity, suggesting the importance of antibody maturation for functionality. Both ADCVI and percent ADCC killing prechallenge were significantly correlated with reduced acute viremia. The latter, as well as postchallenge ADCVI and ADCC, was also significantly correlated with reduced chronic viremia. We have previously demonstrated induction by the prime/boost regimen of mucosal antibodies that inhibit transcytosis of SIV across an intact epithelial cell layer. Here, antibody in rectal secretions was significantly correlated with transcytosis inhibition. Importantly, the transcytosis specific activity (percent inhibition/total secretory IgA and IgG) was strongly correlated with reduced chronic viremia, suggesting that mucosal antibody may help control cell-to-cell viral spread during the course of infection. Overall, the replicating Ad5hr-HIV/SIV priming/gp140 protein boosting approach elicited strong systemic and mucosal antibodies with multiple functional activities associated with control of both acute and chronic viremia.
Figures

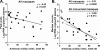
References
-
- Ahmad, R., S. T. Sindhu, E. Toma, R. Morisset, J. Vincelette, J. Menezes, and A. Ahmad. 2001. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J. Clin. Immunol. 21:227-233. - PubMed
-
- Alfsen, A., P. Iniguez, E. Bouguyon, and M. Bomsel. 2001. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J. Immunol. 166:6257-6265. - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. - PubMed
-
- Banks, N. D., N. Kinsey, J. Clements, and J. E. Hildreth. 2002. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS Res. Hum. Retroviruses 18:1197-1205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

