Attenuated Bordetella pertussis protects against highly pathogenic influenza A viruses by dampening the cytokine storm
- PMID: 20444902
- PMCID: PMC2898226
- DOI: 10.1128/JVI.02542-09
Attenuated Bordetella pertussis protects against highly pathogenic influenza A viruses by dampening the cytokine storm
Abstract
The threat of a pandemic spread of highly virulent influenza A viruses currently represents a top global public health problem. Mass vaccination remains the most effective way to combat influenza virus. However, current vaccination strategies face the challenge to meet the demands in a pandemic situation. In a mouse model of severe influenza virus-induced pneumonitis, we observed that prior nasal administration of an attenuated strain of Bordetella pertussis (BPZE1) provided effective and sustained protection against lethal challenge with two different influenza A virus subtypes. In contrast to most cross-protective effects reported so far, the protective window offered upon nasal treatment with BPZE1 lasted up to at least 12 weeks, suggesting a unique mechanism(s) involved in the protection. No significant differences in viral loads were observed between BPZE1-treated and control mice, indicating that the cross-protective mechanism(s) does not directly target the viral particles and/or infected cells. This was further confirmed by the absence of cross-reactive antibodies and T cells in serum transfer and in vitro restimulation experiments, respectively. Instead, compared to infected control mice, BPZE1-treated animals displayed markedly reduced lung inflammation and tissue damage, decreased neutrophil infiltration, and strong suppression of the production of major proinflammatory mediators in their bronchoalveolar fluids (BALFs). Our findings thus indicate that protection against influenza virus-induced severe pneumonitis can be achieved through attenuation of exaggerated cytokine-mediated inflammation. Furthermore, nasal treatment with live attenuated B. pertussis offers a potential alternative to conventional approaches in the fight against one of the most frightening current global public health threats.
Figures

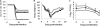


Similar articles
-
Immunogenicity of live attenuated B. pertussis BPZE1 producing the universal influenza vaccine candidate M2e.PLoS One. 2013;8(3):e59198. doi: 10.1371/journal.pone.0059198. Epub 2013 Mar 19. PLoS One. 2013. PMID: 23555631 Free PMC article.
-
Dual mechanism of protection by live attenuated Bordetella pertussis BPZE1 against Bordetella bronchiseptica in mice.Vaccine. 2012 Aug 31;30(40):5864-70. doi: 10.1016/j.vaccine.2012.07.005. Epub 2012 Jul 17. Vaccine. 2012. PMID: 22814407
-
Vaccination with Recombinant Parainfluenza Virus 5 Expressing Neuraminidase Protects against Homologous and Heterologous Influenza Virus Challenge.J Virol. 2017 Nov 14;91(23):e01579-17. doi: 10.1128/JVI.01579-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28931689 Free PMC article.
-
Non-specific Effects of Live Attenuated Pertussis Vaccine Against Heterologous Infectious and Inflammatory Diseases.Front Immunol. 2018 Dec 7;9:2872. doi: 10.3389/fimmu.2018.02872. eCollection 2018. Front Immunol. 2018. PMID: 30581436 Free PMC article. Review.
-
[Tetravaccine--new fundamental approach to prevention of influenza pandemic].Zh Mikrobiol Epidemiol Immunobiol. 2007 Jul-Aug;(4):15-9. Zh Mikrobiol Epidemiol Immunobiol. 2007. PMID: 17882832 Review. Russian.
Cited by
-
Cancer vs. SARS-CoV-2 induced inflammation, overlapping functions, and pharmacological targeting.Inflammopharmacology. 2021 Apr;29(2):343-366. doi: 10.1007/s10787-021-00796-w. Epub 2021 Mar 15. Inflammopharmacology. 2021. PMID: 33723711 Free PMC article. Review.
-
Intranasal Immunization with the Influenza A Virus Encoding Truncated NS1 Protein Protects Mice from Heterologous Challenge by Restraining the Inflammatory Response in the Lungs.Microorganisms. 2021 Mar 26;9(4):690. doi: 10.3390/microorganisms9040690. Microorganisms. 2021. PMID: 33810549 Free PMC article.
-
Commensal Microbes Affect Host Humoral Immunity to Bordetella pertussis Infection.Infect Immun. 2019 Sep 19;87(10):e00421-19. doi: 10.1128/IAI.00421-19. Print 2019 Oct. Infect Immun. 2019. PMID: 31308086 Free PMC article.
-
Attenuated Bordetella pertussis vaccine protects against respiratory syncytial virus disease via an IL-17-dependent mechanism.Am J Respir Crit Care Med. 2014 Jan 15;189(2):194-202. doi: 10.1164/rccm.201307-1227OC. Am J Respir Crit Care Med. 2014. PMID: 24261996 Free PMC article.
-
Geldanamycin Reduces Acute Respiratory Distress Syndrome and Promotes the Survival of Mice Infected with the Highly Virulent H5N1 Influenza Virus.Front Cell Infect Microbiol. 2017 Jun 15;7:267. doi: 10.3389/fcimb.2017.00267. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28664154 Free PMC article.
References
-
- Abe, T., H. Takahashi, H. Hamazaki, N. Miyano-Kurosaki, Y. Matsuura, and H. Takaku. 2003. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J. Immunol. 171:1133-1139. - PubMed
-
- Bao, Z., S. Lim, W. Liao, Y. Lin, C. Thiemermann, B. P. Leung, and W. S. F. Wong. 2007. Glycogen synthase kinase-3beta inhibition attenuates asthma in mice. Am. J. Respir. Crit. Care Med. 176:431-438. - PubMed
-
- Beigel, J. H., J. Farrar, A. M. Han, F. G. Hayden, R. Hyer, M. D. de Jong, S. Lochindarat, T. K. Nguyen, T. H. Nguyen, T. H. Tran, A. Nicoll, S. Touch, K. Y. Yuen, and Writing Committee of the World Health Organization (WHO) Consultation on Human Influenza A/H5. 2005. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 353:1374-1385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

