Clinical, genetic, and functional characterization of four patients carrying partial loss-of-function mutations in the steroidogenic acute regulatory protein (StAR)
- PMID: 20444910
- PMCID: PMC2928910
- DOI: 10.1210/jc.2010-0437
Clinical, genetic, and functional characterization of four patients carrying partial loss-of-function mutations in the steroidogenic acute regulatory protein (StAR)
Erratum in
- J Clin Endocrinol Metab. 2011 Dec;96(12):3908
Abstract
Context: Nonclassic congenital lipoid adrenal hyperplasia (lipoid CAH) is a recently recognized disorder caused by mutations in the steroidogenic acute regulatory protein (StAR) that retain partial function. Affected individuals can present with a phenotype of late onset adrenal insufficiency with only mild or minimally disordered sexual development.
Objectives: The aim was to delineate the clinical spectrum of StAR mutations and correlate phenotype with StAR activity.
Patients: Four patients had nonclassic/atypical lipoid CAH. Adrenal insufficiency was manifested at birth in two patients and at 11 months and 4 yr in the other two. Three were 46,XY with underdeveloped genitalia.
Methods: The StAR gene was sequenced, mutations were recreated in expression vectors, and StAR activity was measured as pregnenolone production in COS-1 cells cotransfected with the cholesterol side-chain cleavage system. StAR mutants were expressed as N-62 StAR in bacteria, and purified proteins were tested for activity with isolated steroidogenic mitochondria and for cholesterol-binding capacity.
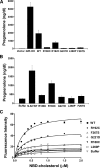
Results: DNA sequencing identified mutations on all alleles. Missense mutations were R188C, G221D, L260P, and F267S; we also tested R192C described by others. The respective activities of R188C, R192C, G221D, L260P, and F267S were 8.0, 39.4, 2.4, 3.1, and 6.1% of wild-type in transfected cells, and 12.8, 54.8, 6.3, 1.8, and 9.5% with isolated mitochondria. Cholesterol binding capacities of R188C, R192C, G221D, L260P, and F267S were 6.7, 55.3, 10.2, 4.6, and 20.9%. These data are correlated to the three-dimensional structure of StAR.
Conclusions: There is a broad clinical spectrum of StAR mutations; StAR activities in vitro correlate well with clinical phenotypes.
Figures

Similar articles
-
Partial defect in the cholesterol side-chain cleavage enzyme P450scc (CYP11A1) resembling nonclassic congenital lipoid adrenal hyperplasia.J Clin Endocrinol Metab. 2011 Mar;96(3):792-8. doi: 10.1210/jc.2010-1828. Epub 2010 Dec 15. J Clin Endocrinol Metab. 2011. PMID: 21159840 Free PMC article.
-
Characterization of novel StAR (steroidogenic acute regulatory protein) mutations causing non-classic lipoid adrenal hyperplasia.PLoS One. 2011;6(5):e20178. doi: 10.1371/journal.pone.0020178. Epub 2011 May 27. PLoS One. 2011. PMID: 21647419 Free PMC article.
-
Congenital lipoid adrenal hyperplasia: functional characterization of three novel mutations in the STAR gene.J Clin Endocrinol Metab. 2010 Mar;95(3):1301-8. doi: 10.1210/jc.2009-1176. Epub 2010 Jan 15. J Clin Endocrinol Metab. 2010. PMID: 20080861
-
STAR splicing mutations cause the severe phenotype of lipoid congenital adrenal hyperplasia: insights from a novel splice mutation and review of reported cases.Clin Endocrinol (Oxf). 2014 Feb;80(2):191-9. doi: 10.1111/cen.12293. Epub 2013 Aug 17. Clin Endocrinol (Oxf). 2014. PMID: 23859637 Review.
-
Clinical spectrum of human STAR variants and their genotype-phenotype correlation.J Endocrinol. 2024 Jul 18;262(3):e240078. doi: 10.1530/JOE-24-0078. Print 2024 Sep 1. J Endocrinol. 2024. PMID: 38913505 Review.
Cited by
-
New Insight into Pathogenic Variant p.L275P on the Structure and Function of Steroidogenic Acute Regulatory Protein (STAR) Through Molecular Dynamics Simulation.Adv Biomed Res. 2025 May 31;14:46. doi: 10.4103/abr.abr_439_23. eCollection 2025. Adv Biomed Res. 2025. PMID: 40519569 Free PMC article.
-
Alterations in polyadenylation and its implications for endocrine disease.Front Endocrinol (Lausanne). 2013 May 8;4:53. doi: 10.3389/fendo.2013.00053. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 23658553 Free PMC article.
-
Partial defect in the cholesterol side-chain cleavage enzyme P450scc (CYP11A1) resembling nonclassic congenital lipoid adrenal hyperplasia.J Clin Endocrinol Metab. 2011 Mar;96(3):792-8. doi: 10.1210/jc.2010-1828. Epub 2010 Dec 15. J Clin Endocrinol Metab. 2011. PMID: 21159840 Free PMC article.
-
Congenital lipoid adrenal hyperplasia (a rare form of adrenal insufficiency and ambiguous genitalia) caused by a novel mutation of the steroidogenic acute regulatory protein gene.Eur J Pediatr. 2012 May;171(5):787-93. doi: 10.1007/s00431-011-1620-5. Epub 2011 Nov 15. Eur J Pediatr. 2012. PMID: 22083155
-
Ovarian cyst torsion in a patient with congenital lipoid adrenal hyperplasia.Eur J Pediatr. 2011 Apr;170(4):535-8. doi: 10.1007/s00431-010-1342-0. Epub 2010 Nov 6. Eur J Pediatr. 2011. PMID: 21057961
References
-
- Miller WL 1997 Congenital lipoid adrenal hyperplasia: the human gene knockout for the steroidogenic acute regulatory protein. J Mol Endocrinol 19:227–240 - PubMed
-
- Matteson KJ, Chung BC, Urdea MS, Miller WL 1986 Study of cholesterol side-chain cleavage (20,22 desmolase) deficiency causing congenital lipoid adrenal hyperplasia using bovine-sequence P450scc oligodeoxyribonucleotide probes. Endocrinology 118:1296–1305 - PubMed
-
- Lin D, Sugawara T, Strauss 3rd JF, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL 1995 Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science 267:1828–1831 - PubMed
-
- Tee MK, Lin D, Sugawara T, Holt JA, Guiguen Y, Buckingham B, Strauss 3rd JF, Miller WL 1995 T→A transversion 11 bp from a splice acceptor site in the human gene for steroidogenic acute regulatory protein causes congenital lipoid adrenal hyperplasia. Hum Mol Genet 4:2299–2305 - PubMed

