Multicenter study to determine disk diffusion and broth microdilution criteria for prediction of high- and low-level mupirocin resistance in Staphylococcus aureus
- PMID: 20444971
- PMCID: PMC2897470
- DOI: 10.1128/JCM.00340-10
Multicenter study to determine disk diffusion and broth microdilution criteria for prediction of high- and low-level mupirocin resistance in Staphylococcus aureus
Abstract
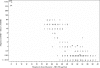
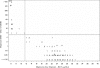
Mupirocin susceptibility testing of Staphylococcus aureus has become more important as mupirocin is used more widely to suppress or eliminate S. aureus colonization and prevent subsequent health care- and community-associated infections. The present multicenter study evaluated two susceptibility testing screening methods to detect mupirocin high-level resistance (HLR), broth microdilution (BMD) MICs of >or=512 microg/ml, and a 6-mm zone diameter for a disk diffusion (DD) test with a 200-microg disk. Initial testing indicated that with Clinical and Laboratory Standards Institute methods for BMD and DD testing, the optimal conditions for the detection of mupirocin HLR were 24 h of incubation and reading of the DD zone diameters with transmitted light. Using the presence or absence of mupA as the "gold standard" for HLR, the sensitivity and specificity of a single-well 256 microg/ml BMD test were 97 and 99%, respectively, and those for the 200-microg disk test were 98 and 99%, respectively. Testing with two disks, 200 microg and 5 microg, was evaluated for its ability to distinguish HLR isolates (MICs >or= 512 microg/ml), low-level-resistant (LLR) isolates (MICs = 8 to 256 microg/ml), and susceptible isolates (MICs <or= 4 microg/ml). Using no zone with both disks as an indication of HLR and no zone with the 5-microg disk plus any zone with the 200-microg disk as LLR, only 3 of the 340 isolates were misclassified, with 3 susceptible isolates being classified as LLR. Use of standardized MIC or disk tests could enable the detection of emerging high- and low-level mupirocin resistance in S. aureus.
Figures
References
-
- Clinical and Laboratory Standards Institute/NCCLS. 2008. Development of in vitro susceptibility testing criteria and quality control parameters; approved guideline, 3rd ed. CLSI document M23-A3. Clinical and Laboratory Standards Institute, Wayne, PA.
-
- Clinical and Laboratory Standards Institute/NCCLS. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed. CLSI document M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA.
-
- Clinical and Laboratory Standards Institute/NCCLS. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. CLSI document M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
-
- Clinical and Laboratory Standards Institute/NCCLS. 2006. Performance standards for antimicrobial disk susceptibility tests; approved standard, 9th ed. CLSI document M2-A9. Clinical and Laboratory Standards Institute, Wayne, PA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

