Rapid lytic granule convergence to the MTOC in natural killer cells is dependent on dynein but not cytolytic commitment
- PMID: 20444980
- PMCID: PMC2893988
- DOI: 10.1091/mbc.e09-11-0930
Rapid lytic granule convergence to the MTOC in natural killer cells is dependent on dynein but not cytolytic commitment
Abstract
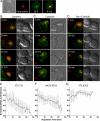
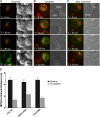
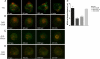
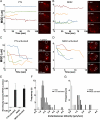
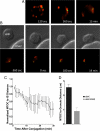
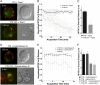
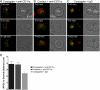
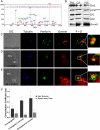
Natural killer cells are lymphocytes specialized to participate in host defense through their innate ability to mediate cytotoxicity by secreting the contents of preformed secretory lysosomes (lytic granules) directly onto a target cell. This form of directed secretion requires the formation of an immunological synapse and occurs stepwise with actin reorganization preceding microtubule-organizing center (MTOC) polarization to the synapse. Because MTOC polarization to the synapse is required for polarization of lytic granules, we attempted to define their interrelationship. We found that compared with the time required for MTOC polarization, lytic granules converged to the MTOC rapidly. The MTOC-directed movement of lytic granules was independent of actin and microtubule reorganization, dependent on dynein motor function, occurred before MTOC polarization, and did not require a commitment to cytotoxicity. This defines a novel paradigm for rapid MTOC-directed transport as a prerequisite for directed secretion, one that may prepare, but not commit cells for precision secretory function.
Figures
References
-
- Barber D. F., Faure M., Long E. O. LFA-1 contributes an early signal for NK cell cytotoxicity. J. Immunol. 2004;173:3653–3659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

