Subthreshold membrane conductances enhance directional selectivity in vertebrate sensory neurons
- PMID: 20445028
- PMCID: PMC4850070
- DOI: 10.1152/jn.01113.2009
Subthreshold membrane conductances enhance directional selectivity in vertebrate sensory neurons
Abstract
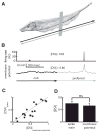
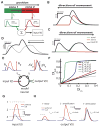
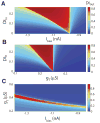
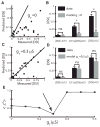
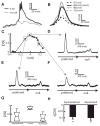
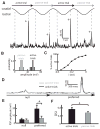
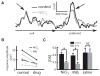
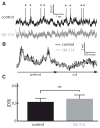
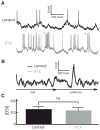
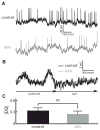
Directional selectivity, in which neurons respond preferentially to one "preferred" direction of movement over the opposite "null" direction, is a critical computation that is found in the central nervous systems of many animals. Such responses are generated using two mechanisms: spatiotemporal convergence via pathways that differ in the timing of information from different locations on the receptor array and the nonlinear integration of this information. Previous studies have showed that various mechanisms may act as nonlinear integrators by suppressing the response in the null direction. Here we show, through a combination of mathematical modeling and in vivo intracellular recordings, that subthreshold membrane conductances can act as a nonlinear integrator by increasing the response in the preferred direction of motion only, thereby enhancing the directional bias. Such subthreshold conductances are ubiquitous in the CNS and therefore may be used in a wide array of computations that involve the enhancement of an existing bias arising from differential spatiotemporal filtering.
Conflict of interest statement
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures
Similar articles
-
Differences in the time course of short-term depression across receptive fields are correlated with directional selectivity in electrosensory neurons.J Neurophysiol. 2009 Dec;102(6):3270-9. doi: 10.1152/jn.00645.2009. Epub 2009 Sep 30. J Neurophysiol. 2009. PMID: 19793877 Free PMC article.
-
Stimulus selectivity is enhanced by voltage-dependent conductances in combination-sensitive neurons.J Neurophysiol. 2006 Dec;96(6):3362-77. doi: 10.1152/jn.00839.2006. Epub 2006 Sep 27. J Neurophysiol. 2006. PMID: 17005607
-
Bursts and isolated spikes code for opposite movement directions in midbrain electrosensory neurons.PLoS One. 2012;7(6):e40339. doi: 10.1371/journal.pone.0040339. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768279 Free PMC article.
-
Orientation in the dark: brain circuits involved in the perception of electric signals in mormyrid electric fish.Eur J Morphol. 1999 Apr;37(2-3):200-5. doi: 10.1076/ejom.37.2.200.4747. Eur J Morphol. 1999. PMID: 10342457 Review.
-
Ionic and neuromodulatory regulation of burst discharge controls frequency tuning.J Physiol Paris. 2008 Jul-Nov;102(4-6):195-208. doi: 10.1016/j.jphysparis.2008.10.019. Epub 2008 Oct 18. J Physiol Paris. 2008. PMID: 18992813 Free PMC article. Review.
Cited by
-
Coding movement direction by burst firing in electrosensory neurons.J Neurophysiol. 2011 Oct;106(4):1954-68. doi: 10.1152/jn.00116.2011. Epub 2011 Jul 20. J Neurophysiol. 2011. PMID: 21775723 Free PMC article.
-
Electrosensory Midbrain Neurons Display Feature Invariant Responses to Natural Communication Stimuli.PLoS Comput Biol. 2015 Oct 16;11(10):e1004430. doi: 10.1371/journal.pcbi.1004430. eCollection 2015 Oct. PLoS Comput Biol. 2015. PMID: 26474395 Free PMC article.
-
Neural Synchrony Gives Rise to Amplitude- and Duration-Invariant Encoding Consistent With Perception of Natural Communication Stimuli.Front Neurosci. 2020 Feb 5;14:79. doi: 10.3389/fnins.2020.00079. eCollection 2020. Front Neurosci. 2020. PMID: 32116522 Free PMC article.
-
Efficient computation via sparse coding in electrosensory neural networks.Curr Opin Neurobiol. 2011 Oct;21(5):752-60. doi: 10.1016/j.conb.2011.05.016. Epub 2011 Jun 16. Curr Opin Neurobiol. 2011. PMID: 21683574 Free PMC article. Review.
-
Burst firing optimizes invariant coding of natural communication signals by electrosensory neural populations.iScience. 2025 Apr 9;28(5):112399. doi: 10.1016/j.isci.2025.112399. eCollection 2025 May 16. iScience. 2025. PMID: 40641556 Free PMC article.
References
-
- Adelson EH, Bergen JR. Spatiotemporal energy models for the perception of motion. J Opt Soc Am A Opt Image Sci. 1985;2:284–299. - PubMed
-
- Bastian J. Electrolocation. II. The effects of moving objects and other electrical stimuli on the activities of two categories of posterior lateral line lobe cells in Apteronotus albifrons. J Comp Physiol [A] 1981;144:481–494.
-
- Berman N, Dunn RJ, Maler L. Function of NMDA receptors and persistent sodium channels in a feedback pathway of the electrosensory system. J Neurophysiol. 2001;86:1612–1621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

