Taste-specific cell assemblies in a biologically informed model of the nucleus of the solitary tract
- PMID: 20445036
- PMCID: PMC2904217
- DOI: 10.1152/jn.01098.2009
Taste-specific cell assemblies in a biologically informed model of the nucleus of the solitary tract
Abstract
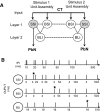
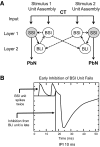
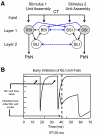
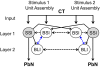
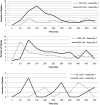
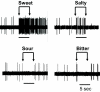
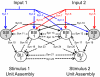
Although the cellular organization of many primary sensory nuclei has been well characterized, questions remain about the functional architecture of the first central relay for gustation, the rostral nucleus of the solitary tract (NTS). Here we used electrophysiological data recorded from single cells in the NTS to inform a network model of taste processing. Previous studies showed that electrical stimulation of the chorda tympani (CT) nerve initiates two types of inhibitory influences with different time courses in separate groups of NTS cells. Each type of inhibition targeted cells with distinct taste response properties. Further analyses of these data identified three NTS cell types differentiated by their latency of evoked response, time course of CT evoked inhibition, and degree of selectivity across taste qualities. Based on these results, we designed a model of the NTS consisting of discrete, reciprocally connected, stimulus-specific "cell" assemblies. Input to the network of integrate-and-fire model neurons was based on electrophysiological recordings from the CT nerve. Following successful simulation of paired-pulse CT stimulation, the network was tested for its ability to discriminate between two "taste" stimuli. Network dynamics of the model produced biologically plausible responses from each unit type and enhanced discrimination between taste qualities. We propose that an interactive network of taste quality specific cell assemblies, similar to our model, may account for the coherence in across-neuron patterns of NTS responses between similar tastants.
Figures



Similar articles
-
Effects of electrical stimulation of the chorda tympani nerve on taste responses in the nucleus of the solitary tract.J Neurophysiol. 2002 Nov;88(5):2477-89. doi: 10.1152/jn.00094.2002. J Neurophysiol. 2002. PMID: 12424287
-
Two types of inhibitory influences target different groups of taste-responsive cells in the nucleus of the solitary tract of the rat.Brain Res. 2009 Jun 12;1275:24-32. doi: 10.1016/j.brainres.2009.03.069. Epub 2009 Apr 14. Brain Res. 2009. PMID: 19371730 Free PMC article.
-
Transfer of information about taste from the nucleus of the solitary tract to the parabrachial nucleus of the pons.Brain Res. 1997 Jul 25;763(2):167-81. doi: 10.1016/s0006-8993(97)00217-5. Brain Res. 1997. PMID: 9296557
-
Neural circuits for taste. Excitation, inhibition, and synaptic plasticity in the rostral gustatory zone of the nucleus of the solitary tract.Ann N Y Acad Sci. 1998 Nov 30;855:467-74. doi: 10.1111/j.1749-6632.1998.tb10607.x. Ann N Y Acad Sci. 1998. PMID: 9929640 Review.
-
Neural plasticity in the gustatory system.Nutr Rev. 2004 Nov;62(11 Pt 2):S208-17; discussion S224-41. doi: 10.1111/j.1753-4887.2004.tb00101.x. Nutr Rev. 2004. PMID: 15630937 Free PMC article. Review.
Cited by
-
A computational analysis of signal fidelity in the rostral nucleus of the solitary tract.J Neurophysiol. 2018 Mar 1;119(3):771-785. doi: 10.1152/jn.00624.2017. Epub 2017 Nov 1. J Neurophysiol. 2018. PMID: 29093172 Free PMC article.
-
Neural coding of taste by simultaneously recorded cells in the nucleus of the solitary tract of the rat.J Neurophysiol. 2012 Dec;108(12):3301-12. doi: 10.1152/jn.00566.2012. Epub 2012 Sep 26. J Neurophysiol. 2012. PMID: 23019002 Free PMC article.
-
Functional MRI activation of the nucleus tractus solitarius after taste stimuli at ultra-high field: a proof-of-concept single-subject study.Front Nutr. 2023 Oct 26;10:1173316. doi: 10.3389/fnut.2023.1173316. eCollection 2023. Front Nutr. 2023. PMID: 37955018 Free PMC article.
References
-
- Adachi M, Oshima T, Yamada S, Satoh T. Corss-correlation analysis of taste neuron pairs in rat solitary tract nucleus. J Neurophysiol 62: 501–509, 1989 - PubMed
-
- Bradley RM. rNST circuits. In: The Role of the Nucleus of the Solitary Tract in Gustatory Processing, edited by Bradley RM. Boca Raton, FL: CRC, 2007 - PubMed
-
- Bradley RM, Grabauskas G. Neural circuits for taste. Excitation, inhibition, and synaptic plasticity in the rostral gustatory zone of the nucleus of the solitary tract. Ann NY Acad Sci 855: 467–474, 1998 - PubMed
-
- Bradley RM, Mistretta CM, Bates CA, Killackey HP. Transganglionic transport of HRP from the circumvallate papilla of the rat. Brain Res 361: 154–161, 1985 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

