Alterations in the hippocampal endocannabinoid system in diet-induced obese mice
- PMID: 20445053
- PMCID: PMC3636535
- DOI: 10.1523/JNEUROSCI.2648-09.2010
Alterations in the hippocampal endocannabinoid system in diet-induced obese mice
Abstract
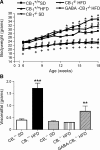
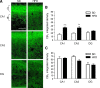
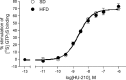
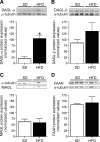
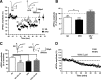
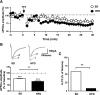
The endocannabinoid (eCB) system plays central roles in the regulation of food intake and energy expenditure. Its alteration in activity contributes to the development and maintenance of obesity. Stimulation of the cannabinoid receptor type 1 (CB(1) receptor) increases feeding, enhances reward aspects of eating, and promotes lipogenesis, whereas its blockade decreases appetite, sustains weight loss, increases insulin sensitivity, and alleviates dysregulation of lipid metabolism. The hypothesis has been put forward that the eCB system is overactive in obesity. Hippocampal circuits are not directly involved in the neuronal control of food intake and appetite, but they play important roles in hedonic aspects of eating. We investigated the possibility whether or not diet-induced obesity (DIO) alters the functioning of the hippocampal eCB system. We found that levels of the two eCBs, 2-arachidonoyl glycerol (2-AG) and anandamide, were increased in the hippocampus from DIO mice, with a concomitant increase of the 2-AG synthesizing enzyme diacylglycerol lipase-alpha and increased CB(1) receptor immunoreactivity in CA1 and CA3 regions, whereas CB(1) receptor agonist-induced [(35)S]GTPgammaS binding was unchanged. eCB-mediated synaptic plasticity was changed in the CA1 region, as depolarization-induced suppression of inhibition and long-term depression of inhibitory synapses were enhanced. Functionality of CB(1) receptors in GABAergic neurons was furthermore revealed, as mice specifically lacking CB(1) receptors on this neuronal population were partly resistant to DIO. Our results show that DIO-induced changes in the eCB system affect not only tissues directly involved in the metabolic regulation but also brain regions mediating hedonic aspects of eating and influencing cognitive processes.
Figures

Similar articles
-
A novel non-CB1/TRPV1 endocannabinoid-mediated mechanism depresses excitatory synapses on hippocampal CA1 interneurons.Hippocampus. 2012 Feb;22(2):209-21. doi: 10.1002/hipo.20884. Epub 2010 Nov 10. Hippocampus. 2012. PMID: 21069781 Free PMC article.
-
Endocannabinoid dysregulation in the pancreas and adipose tissue of mice fed with a high-fat diet.Obesity (Silver Spring). 2008 Mar;16(3):553-65. doi: 10.1038/oby.2007.106. Epub 2008 Jan 17. Obesity (Silver Spring). 2008. PMID: 18239598
-
Molecular reorganization of endocannabinoid signalling in Alzheimer's disease.Brain. 2011 Apr;134(Pt 4):1041-60. doi: 10.1093/brain/awr046. Brain. 2011. PMID: 21459826 Free PMC article.
-
Neurobiological Interactions Between Stress and the Endocannabinoid System.Neuropsychopharmacology. 2016 Jan;41(1):80-102. doi: 10.1038/npp.2015.166. Epub 2015 Jun 12. Neuropsychopharmacology. 2016. PMID: 26068727 Free PMC article. Review.
-
The endocannabinoid-CB receptor system: Importance for development and in pediatric disease.Neuro Endocrinol Lett. 2004 Feb-Apr;25(1-2):24-30. Neuro Endocrinol Lett. 2004. PMID: 15159678 Review.
Cited by
-
A Lower Olfactory Capacity Is Related to Higher Circulating Concentrations of Endocannabinoid 2-Arachidonoylglycerol and Higher Body Mass Index in Women.PLoS One. 2016 Feb 5;11(2):e0148734. doi: 10.1371/journal.pone.0148734. eCollection 2016. PLoS One. 2016. PMID: 26849214 Free PMC article. Clinical Trial.
-
AAV-Mediated Overexpression of the CB1 Receptor in the mPFC of Adult Rats Alters Cognitive Flexibility, Social Behavior, and Emotional Reactivity.Front Behav Neurosci. 2011 Jul 13;5:37. doi: 10.3389/fnbeh.2011.00037. eCollection 2011. Front Behav Neurosci. 2011. PMID: 21808613 Free PMC article.
-
A runner's high depends on cannabinoid receptors in mice.Proc Natl Acad Sci U S A. 2015 Oct 20;112(42):13105-8. doi: 10.1073/pnas.1514996112. Epub 2015 Oct 5. Proc Natl Acad Sci U S A. 2015. PMID: 26438875 Free PMC article.
-
Care and feeding of the endocannabinoid system: a systematic review of potential clinical interventions that upregulate the endocannabinoid system.PLoS One. 2014 Mar 12;9(3):e89566. doi: 10.1371/journal.pone.0089566. eCollection 2014. PLoS One. 2014. PMID: 24622769 Free PMC article.
-
The Endocannabinoid System: Implications in Gastrointestinal Physiology and Pathology.Int J Mol Sci. 2025 Feb 3;26(3):1306. doi: 10.3390/ijms26031306. Int J Mol Sci. 2025. PMID: 39941074 Free PMC article. Review.
References
-
- Bellocchio L, Lafenêtre P, Cannich A, Cota D, Puente N, Grandes P, Chaouloff F, Piazza PV, Marsicano G. Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci. 2010;13:281–283. - PubMed
-
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urbán GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. - PubMed
-
- Breivogel CS, Childers SR. Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J Pharmacol Exp Ther. 2000;295:328–336. - PubMed
-
- Buettner R, Schölmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity. 2007;15:798–808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
