Mammalian target of rapamycin signaling modulates photic entrainment of the suprachiasmatic circadian clock
- PMID: 20445056
- PMCID: PMC2896874
- DOI: 10.1523/JNEUROSCI.5482-09.2010
Mammalian target of rapamycin signaling modulates photic entrainment of the suprachiasmatic circadian clock
Abstract
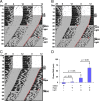
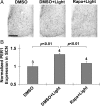
Inducible gene expression appears to be an essential event that couples light to entrainment of the master mammalian circadian clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Recently, we reported that light triggers phase-dependent activation of the mammalian target of rapamycin (mTOR) signaling pathway, a major regulator of protein synthesis, in the SCN, thus raising the possibility that mTOR-evoked mRNA translation contributes to clock entrainment. Here, we used a combination of cellular, molecular, and behavioral assays to address this question. To this end, we show that the in vivo infusion of the mTOR inhibitor rapamycin led to a significant attenuation of the phase-delaying effect of early-night light. Conversely, disruption of mTOR during the late night augmented the phase-advancing effect of light. To assess the role of mTOR signaling within the context of molecular entrainment, the effects of rapamycin on light-induced expression of PERIOD1 and PERIOD2 were examined. At both the early- and late-night time points, abrogation of mTOR signaling led to a significant attenuation of light-evoked PERIOD protein expression. Our results also reveal that light-induced mTOR activation leads to the translation of mRNAs with a 5'-terminal oligopyrimidine tract such as eukaryotic elongation factor 1A and the immediate early gene JunB. Together, these data indicate that the mTOR pathway functions as potent and selective regulator of light-evoked protein translation and SCN clock entrainment.
Figures









Similar articles
-
Translational control of entrainment and synchrony of the suprachiasmatic circadian clock by mTOR/4E-BP1 signaling.Neuron. 2013 Aug 21;79(4):712-24. doi: 10.1016/j.neuron.2013.06.026. Neuron. 2013. PMID: 23972597 Free PMC article.
-
Circadian regulation of mammalian target of rapamycin signaling in the mouse suprachiasmatic nucleus.Neuroscience. 2011 May 5;181:79-88. doi: 10.1016/j.neuroscience.2011.03.005. Epub 2011 Mar 5. Neuroscience. 2011. PMID: 21382453 Free PMC article.
-
Photic regulation of the mTOR signaling pathway in the suprachiasmatic circadian clock.Mol Cell Neurosci. 2008 Jul;38(3):312-24. doi: 10.1016/j.mcn.2008.03.005. Epub 2008 Mar 25. Mol Cell Neurosci. 2008. PMID: 18468454 Free PMC article.
-
Let there be light: signal transduction in a mammalian circadian system.Braz J Med Biol Res. 1996 Jan;29(1):131-40. Braz J Med Biol Res. 1996. PMID: 8731343 Review.
-
Misalignment of Circadian Rhythms in Diet-Induced Obesity.Adv Exp Med Biol. 2024;1460:27-71. doi: 10.1007/978-3-031-63657-8_2. Adv Exp Med Biol. 2024. PMID: 39287848 Review.
Cited by
-
A Symphony of Signals: Intercellular and Intracellular Signaling Mechanisms Underlying Circadian Timekeeping in Mice and Flies.Int J Mol Sci. 2019 May 13;20(9):2363. doi: 10.3390/ijms20092363. Int J Mol Sci. 2019. PMID: 31086044 Free PMC article. Review.
-
New insights into non-transcriptional regulation of mammalian core clock proteins.J Cell Sci. 2020 Sep 15;133(18):jcs241174. doi: 10.1242/jcs.241174. J Cell Sci. 2020. PMID: 32934011 Free PMC article. Review.
-
The paradoxical lean phenotype of hypothyroid mice is marked by increased adaptive thermogenesis in the skeletal muscle.Proc Natl Acad Sci U S A. 2020 Sep 8;117(36):22544-22551. doi: 10.1073/pnas.2008919117. Epub 2020 Aug 21. Proc Natl Acad Sci U S A. 2020. PMID: 32826330 Free PMC article.
-
Emerging roles for post-transcriptional regulation in circadian clocks.Nat Neurosci. 2013 Nov;16(11):1544-50. doi: 10.1038/nn.3543. Epub 2013 Oct 28. Nat Neurosci. 2013. PMID: 24165681 Review.
-
mTOR Senses Intracellular pH through Lysosome Dispersion from RHEB.Bioessays. 2019 Jul;41(7):e1800265. doi: 10.1002/bies.201800265. Epub 2019 Jun 3. Bioessays. 2019. PMID: 31157925 Free PMC article.
References
-
- Adams J, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. - PubMed
-
- Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci. 1999;19:1115–1121. - PMC - PubMed
-
- Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. - PubMed
-
- Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. mPer1 and mper2 are essential for normal resetting of the circadian clock. J Biol Rhythms. 2001;16:100–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
