Functional identification of a pulvinar path from superior colliculus to cortical area MT
- PMID: 20445060
- PMCID: PMC2919315
- DOI: 10.1523/JNEUROSCI.6176-09.2010
Functional identification of a pulvinar path from superior colliculus to cortical area MT
Abstract
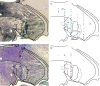
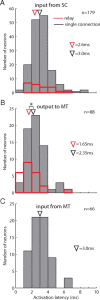
The idea of a second visual pathway, in which visual signals travel from brainstem to cortex via the pulvinar thalamus, has had considerable influence as an alternative to the primary geniculo-striate pathway. Existence of this second pathway in primates, however, is not well established. A major question centers on whether the pulvinar acts as a relay, particularly in the path from the superior colliculus (SC) to the motion area in middle temporal cortex (MT). We used physiological microstimulation to identify pulvinar neurons belonging to the path from SC to MT in the macaque. We made three salient observations. First, we identified many neurons in the visual pulvinar that received input from SC or projected to MT, as well as a largely separate set of neurons that received input from MT. Second, and more importantly, we identified a subset of neurons as relay neurons that both received SC input and projected to MT. The identification of these relay neurons demonstrates a continuous functional path from SC to MT through the pulvinar in primates. Third, we histologically localized a subset of SC-MT relay neurons to the subdivision of inferior pulvinar known to project densely to MT but also localized SC-MT relay neurons to an adjacent subdivision. This pattern indicates that the pulvinar pathway is not limited to a single anatomically defined region. These findings bring new perspective to the functional organization of the pulvinar and its role in conveying signals to the cerebral cortex.
Figures







References
-
- Adams MM, Hof PR, Gattass R, Webster MJ, Ungerleider LG. Visual cortical projections and chemoarchitecture of macaque monkey pulvinar. J Comp Neurol. 2000;419:377–393. - PubMed
-
- Baizer JS, Whitney JF, Bender DB. Bilateral projections from the parabigeminal nucleus to the superior colliculus in monkey. Exp Brain Res. 1991;86:467–470. - PubMed
-
- Baker J, Gibson A, Mower G, Robinson F, Glickstein M. Cat visual corticopontine cells project to the superior colliculus. Brain Res. 1983;265:227–232. - PubMed
-
- Bender DB. Retinotopic organization of macaque pulvinar. J Neurophysiol. 1981;46:672–693. - PubMed
-
- Benevento LA, Fallon JH. The ascending projections of the superior colliculus in the rhesus monkey (Macaca mulatta) J Comp Neurol. 1975;160:339–361. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
