Multiple kinase pathways regulate voltage-dependent Ca2+ influx and migration in oligodendrocyte precursor cells
- PMID: 20445068
- PMCID: PMC2887321
- DOI: 10.1523/JNEUROSCI.5086-09.2010
Multiple kinase pathways regulate voltage-dependent Ca2+ influx and migration in oligodendrocyte precursor cells
Abstract
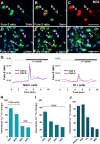
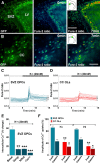
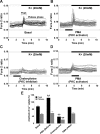
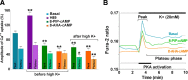
It is becoming increasingly clear that voltage-operated Ca(2+) channels (VOCCs) play a fundamental role in the development of oligodendrocyte progenitor cells (OPCs). Because direct phosphorylation by different kinases is one of the most important mechanisms involved in VOCC modulation, the aim of this study was to evaluate the participation of serine-threonine kinases and tyrosine kinases (TKs) on Ca(2+) influx mediated by VOCCs in OPCs. Calcium imaging revealed that OPCs exhibited Ca(2+) influx after plasma membrane depolarization via L-type VOCCs. Furthermore, VOCC-mediated Ca(2+) influx declined with OPC differentiation, indicating that VOCCs are developmentally regulated in OPCs. PKC activation significantly increased VOCC activity in OPCs, whereas PKA activation produced the opposite effect. The results also indicated that OPC morphological changes induced by PKC activation were partially mediated by VOCCs. Our data clearly suggest that TKs exert an activating influence on VOCC function in OPCs. Furthermore, using the PDGF response as a model to probe the role of TK receptors (TKr) on OPC Ca(2+) uptake, we found that TKr activation potentiated Ca(2+) influx after membrane depolarization. Interestingly, this TKr modulation of VOCCs appeared to be essential for the PDGF enhancement of OPC migration rate, because cell motility was completely blocked by TKr antagonists, as well as VOCC inhibitors, in migration assays. The present study strongly demonstrates that PKC and TKrs enhance Ca(2+) influx induced by depolarization in OPCs, whereas PKA has an inhibitory effect. These kinases modulate voltage-operated Ca(2+) uptake in OPCs and participate in the modulation of process extension and migration.
Figures


Similar articles
-
Voltage-gated Ca2+ entry promotes oligodendrocyte progenitor cell maturation and myelination in vitro.Exp Neurol. 2015 Mar;265:69-83. doi: 10.1016/j.expneurol.2014.12.012. Epub 2014 Dec 24. Exp Neurol. 2015. PMID: 25542980 Free PMC article.
-
Golli myelin basic proteins regulate oligodendroglial progenitor cell migration through voltage-gated Ca2+ influx.J Neurosci. 2009 May 20;29(20):6663-76. doi: 10.1523/JNEUROSCI.5806-08.2009. J Neurosci. 2009. PMID: 19458236 Free PMC article.
-
Regulation of store-operated and voltage-operated Ca2+ channels in the proliferation and death of oligodendrocyte precursor cells by golli proteins.ASN Neuro. 2009 Apr 14;1(1):e00003. doi: 10.1042/AN20090003. ASN Neuro. 2009. PMID: 19570024 Free PMC article.
-
Voltage-operated Ca(2+) and Na(+) channels in the oligodendrocyte lineage.J Neurosci Res. 2009 Nov 15;87(15):3259-66. doi: 10.1002/jnr.21938. J Neurosci Res. 2009. PMID: 19021296 Review.
-
Redox state as a central modulator of precursor cell function.Ann N Y Acad Sci. 2003 Jun;991:251-71. doi: 10.1111/j.1749-6632.2003.tb07481.x. Ann N Y Acad Sci. 2003. PMID: 12846992 Review.
Cited by
-
Glutamate Signaling via the AMPAR Subunit GluR4 Regulates Oligodendrocyte Progenitor Cell Migration in the Developing Spinal Cord.J Neurosci. 2021 Jun 23;41(25):5353-5371. doi: 10.1523/JNEUROSCI.2562-20.2021. Epub 2021 May 11. J Neurosci. 2021. PMID: 33975920 Free PMC article.
-
Conditional Deletion of the L-Type Calcium Channel Cav1.2 in NG2-Positive Cells Impairs Remyelination in Mice.J Neurosci. 2017 Oct 18;37(42):10038-10051. doi: 10.1523/JNEUROSCI.1787-17.2017. Epub 2017 Sep 12. J Neurosci. 2017. PMID: 28899915 Free PMC article.
-
Roles of Ion Channels in Oligodendrocyte Precursor Cells: From Physiology to Pathology.Int J Mol Sci. 2025 Jul 29;26(15):7336. doi: 10.3390/ijms26157336. Int J Mol Sci. 2025. PMID: 40806469 Free PMC article. Review.
-
NG2 glial cells integrate synaptic input in global and dendritic calcium signals.Elife. 2016 Sep 19;5:e16262. doi: 10.7554/eLife.16262. Elife. 2016. PMID: 27644104 Free PMC article.
-
Voltage-gated Ca2+ entry promotes oligodendrocyte progenitor cell maturation and myelination in vitro.Exp Neurol. 2015 Mar;265:69-83. doi: 10.1016/j.expneurol.2014.12.012. Epub 2014 Dec 24. Exp Neurol. 2015. PMID: 25542980 Free PMC article.
References
-
- Agresti C, D'Urso D, Levi G. Reversible inhibitory effects of interferon-γ and tumor necrosis factor-α on oligodendroglial lineage cell proliferation and differentiation in vitro. Eur J Neurosci. 1996;8:1106–1116. - PubMed
-
- Akopian G, Kressin K, Derouiche A, Steinhäuser C. Identified glial cells in the early postnatal mouse hippocampus display different types of Ca2+ currents. Glia. 1996;17:181–194. - PubMed
-
- Althaus HH, Schröter J, Spoerri P, Schwartz P, Klöppner S, Rohmann A, Neuhoff V. Protein kinase C stimulation enhances the process formation of adult oligodendrocytes and induces proliferation. J Neurosci Res. 1991;29:481–489. - PubMed
-
- Amur-Umarjee S, Phan T, Campagnoni AT. Myelin basic protein mRNA translocation in oligodendrocytes is inhibited by astrocytes in vitro. J Neurosci Res. 1993;36:99–110. - PubMed
-
- Avossa D, Pfeiffer SE. Transient reversion of O4+ GalC− oligodendrocyte progenitor development in response to the phorbol ester TPA. J Neurosci Res. 1993;34:113–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
