Colloquium paper: how grandmother effects plus individual variation in frailty shape fertility and mortality: guidance from human-chimpanzee comparisons
- PMID: 20445089
- PMCID: PMC3024018
- DOI: 10.1073/pnas.0914627107
Colloquium paper: how grandmother effects plus individual variation in frailty shape fertility and mortality: guidance from human-chimpanzee comparisons
Abstract
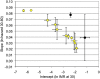
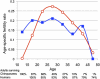
In the first paper to present formal theory explaining that senescence is a consequence of natural selection, W. D. Hamilton concluded that human postmenopausal longevity results from the contributions of ancestral grandmothers to the reproduction of their relatives. A grandmother hypothesis, subsequently elaborated with additional lines of evidence, helps explain both exceptional longevity and additional features of life history that distinguish humans from the other great apes. However, some of the variation observed in aging rates seems inconsistent with the tradeoffs between current and future reproduction identified by theory. In humans and chimpanzees, our nearest living relatives, individuals who bear offspring at faster rates do not cease bearing sooner. They continue to be fertile longer instead. Furthermore, within both species, groups with lower overall mortality rates have faster rates of increase in death risk with advancing age. These apparent contradictions to the expected life history tradeoffs likely result from heterogeneity in frailty among individuals. Whereas robust and frail alike must allocate investments between current and future reproduction, the more robust can afford more of both. This heterogeneity, combined with evolutionary tradeoffs and the key role of ancestral grandmothers they identify, helps explain aspects of human aging that increasingly concern us all.
Conflict of interest statement
The author declares no conflict of interest.
Figures



Similar articles
-
Grandmothers and the evolution of human longevity.Am J Hum Biol. 2003 May-Jun;15(3):380-400. doi: 10.1002/ajhb.10156. Am J Hum Biol. 2003. PMID: 12704714 Review.
-
Mortality and fertility rates in humans and chimpanzees: How within-species variation complicates cross-species comparisons.Am J Hum Biol. 2009 Jul-Aug;21(4):578-86. doi: 10.1002/ajhb.20890. Am J Hum Biol. 2009. PMID: 19213006 Free PMC article.
-
The grandmother effect: implications for studies on aging and cognition.Gerontology. 2010;56(1):73-9. doi: 10.1159/000236045. Epub 2009 Sep 3. Gerontology. 2010. PMID: 19729883 Free PMC article.
-
Grandmothers and the evolution of human longevity: a review of findings and future directions.Evol Anthropol. 2013 Nov-Dec;22(6):294-302. doi: 10.1002/evan.21382. Evol Anthropol. 2013. PMID: 24347503 Review.
-
Increased longevity evolves from grandmothering.Proc Biol Sci. 2012 Dec 22;279(1749):4880-4. doi: 10.1098/rspb.2012.1751. Epub 2012 Oct 24. Proc Biol Sci. 2012. PMID: 23097518 Free PMC article.
Cited by
-
Hugh's book and Krogh's animals: biodiversity and textbook examples in teaching.Adv Physiol Educ. 2014 Sep;38(3):195-8. doi: 10.1152/advan.00042.2014. Adv Physiol Educ. 2014. PMID: 25179607 Free PMC article.
-
Ongoing selection for a uniquely human null allele of SIGLEC12 in world-wide populations may protect against the risk of advanced carcinomas.FASEB Bioadv. 2021 Mar 30;3(4):278-279. doi: 10.1096/fba.2021-00036. eCollection 2021 Apr. FASEB Bioadv. 2021. PMID: 33842853 Free PMC article. No abstract available.
-
Life history impacts on infancy and the evolution of human social cognition.Front Psychol. 2023 Nov 9;14:1197378. doi: 10.3389/fpsyg.2023.1197378. eCollection 2023. Front Psychol. 2023. PMID: 38023007 Free PMC article.
-
Evolution of Human Pair Bonds as a Consequence of Male-Biased Mating Sex Ratios?Bull Math Biol. 2025 Jan 30;87(3):37. doi: 10.1007/s11538-025-01414-4. Bull Math Biol. 2025. PMID: 39883339 Free PMC article.
-
On the apparent rarity of epithelial cancers in captive chimpanzees.Philos Trans R Soc Lond B Biol Sci. 2015 Jul 19;370(1673):20140225. doi: 10.1098/rstb.2014.0225. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26056369 Free PMC article. Review.
References
-
- Oeppen J, Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296:1029–1031. - PubMed
-
- Keyfitz N, Fleiger W. World Population: An Analysis of Vital Data. Chicago: Univ of Chicago Press; 1968.
-
- Howell N. Demography of the Dobe! Kung. New York: Academic Press; 1979.
-
- Hill K, Hurtado AM. Ache Life History: The Ecology and Demography of a Foraging People. New York: de Gruyter; 1996.
-
- Early JD, Headland TN. Population Dynamics of a Philippine Rain Forest People: The San Ildefonso Agta. Gainesville: Univ Press of Florida; 1998.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

