pH-dependent modulation of voltage gating in connexin45 homotypic and connexin45/connexin43 heterotypic gap junctions
- PMID: 20445098
- PMCID: PMC2906847
- DOI: 10.1073/pnas.1004552107
pH-dependent modulation of voltage gating in connexin45 homotypic and connexin45/connexin43 heterotypic gap junctions
Abstract
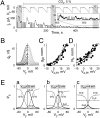
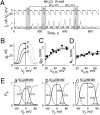
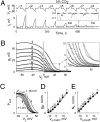
Intracellular pH (pH(i)) can change during physiological and pathological conditions causing significant changes of electrical and metabolic cell-cell communication through gap junction (GJ) channels. In HeLa cells expressing wild-type connexin45 (Cx45) as well as Cx45 and Cx43 tagged with EGFP, we examined how pH(i) affects junctional conductance (g(j)) and g(j) dependence on transjunctional voltage (V(j)). To characterize V(j) gating, we fit the g(j)-V(j) relation using a stochastic four-state model containing one V(j)-sensitive gate in each apposed hemichannel (aHC); aHC open probability was a Boltzmann function of the fraction of V(j) across it. Using the model, we estimated gating parameters characterizing sensitivity to V(j) and number of functional channels. In homotypic Cx45 and heterotypic Cx45/Cx43-EGFP GJs, pH(i) changes from 7.2 to approximately 8.0 shifted g(j)-V(j) dependence of Cx45 aHCs along the V(j) axis resulting in increased probability of GJ channels being in the fully open state without change in the slope of g(j) dependence on V(j). In contrast, acidification shifted g(j)-V(j) dependence in the opposite direction, reducing open probability; acidification also reduced the number of functional channels. Correlation between the number of channels in Cx45-EGFP GJs and maximal g(j) achieved under alkaline conditions showed that only approximately 4% of channels were functional. The acid dissociation constant (pK(a)) of g(j)-pH(i) dependence of Cx45/Cx45 GJs was approximately 7. The pK(a) of heterotypic Cx45/Cx43-EGFP GJs was lower, approximately 6.7, between the pK(a)s of Cx45 and Cx43-EGFP (approximately 6.5) homotypic GJs. In summary, pH(i) significantly modulates junctional conductance of Cx45 by affecting both V(j) gating and number of functional channels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Demaurex N. pH Homeostasis of cellular organelles. News Physiol Sci. 2002;17:1–5. - PubMed
-
- Delmar M, Coombs W, Sorgen P, Duffy HS, Taffet SM. Structural bases for the chemical regulation of connexin43 channels. Cardiovasc Res. 2004;62:268–275. - PubMed
-
- Obara M, Szeliga M, Albrecht J. Regulation of pH in the mammalian central nervous system under normal and pathological conditions: Facts and hypotheses. Neurochem Int. 2008;52:905–919. - PubMed
-
- Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992;15:396–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

