Bioenergetic characterization of mouse podocytes
- PMID: 20445170
- PMCID: PMC2928644
- DOI: 10.1152/ajpcell.00563.2009
Bioenergetic characterization of mouse podocytes
Abstract
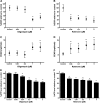
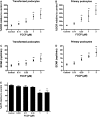
Mitochondrial dysfunction contributes to podocyte injury, but normal podocyte bioenergetics have not been characterized. We measured oxygen consumption rates (OCR) and extracellular acidification rates (ECAR), using a transformed mouse podocyte cell line and the Seahorse Bioscience XF24 Extracellular Flux Analyzer. Basal OCR and ECAR were 55.2 +/- 9.9 pmol/min and 3.1 +/- 1.9 milli-pH units/min, respectively. The complex V inhibitor oligomycin reduced OCR to approximately 45% of baseline rates, indicating that approximately 55% of cellular oxygen consumption was coupled to ATP synthesis. Rotenone, a complex I inhibitor, reduced OCR to approximately 25% of the baseline rates, suggesting that mitochondrial respiration accounted for approximately 75% of the total cellular respiration. Thus approximately 75% of mitochondrial respiration was coupled to ATP synthesis and approximately 25% was accounted for by proton leak. Carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), which uncouples electron transport from ATP generation, increased OCR and ECAR to approximately 360% and 840% of control levels. FCCP plus rotenone reduced ATP content by 60%, the glycolysis inhibitor 2-deoxyglucose reduced ATP by 35%, and 2-deoxyglucose in combination with FCCP or rotenone reduced ATP by >85%. The lactate dehydrogenase inhibitor oxamate and 2-deoxyglucose did not reduce ECAR, and 2-deoxyglucose had no effect on OCR, although 2-deoxyglucose reduced ATP content by 25%. Mitochondrial uncoupling induced by FCCP was associated with increased OCR with certain substrates, including lactate, glucose, pyruvate, and palmitate. Replication of these experiments in primary mouse podocytes yielded similar data. We conclude that mitochondria play the primary role in maintaining podocyte energy homeostasis, while glycolysis makes a lesser contribution.
Figures






References
-
- Brand MD. The efficiency and plasticity of mitochondrial energy transduction. Biochem Soc Trans 33: 897–904, 2005 - PubMed
-
- Brookes PS. Mitochondrial H+ leak and ROS generation: an odd couple. Free Radic Biol Med 38: 12–23, 2005 - PubMed
-
- Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, Caridi G, Piemonte F, Montini G, Ghiggeri GM, Murer L, Barisoni L, Pastore A, Muda AO, Valente ML, Bertini E, Emma F. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol 18: 2773–2780, 2007 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

