PKC-delta and -eta, MEKK-1, MEK-6, MEK-3, and p38-delta are essential mediators of the response of normal human epidermal keratinocytes to differentiating agents
- PMID: 20445555
- PMCID: PMC3120227
- DOI: 10.1038/jid.2010.108
PKC-delta and -eta, MEKK-1, MEK-6, MEK-3, and p38-delta are essential mediators of the response of normal human epidermal keratinocytes to differentiating agents
Erratum in
-
Corrigendum to "PKC-δ and -η, MEKK-1, MEK-6, MEK-3, and p38-δ Are Essential Mediators of the Response of Normal Human Epidermal Keratinocytes to Differentiating Agents" [Journal of Investigative Dermatology, Volume 130, Issue 8, August 2010, Pages 2017-2030].J Invest Dermatol. 2025 Jun;145(6):1542-1543. doi: 10.1016/j.jid.2024.12.001. Epub 2025 Apr 19. J Invest Dermatol. 2025. PMID: 40252056 No abstract available.
Abstract
Previous studies suggest that the novel protein kinase C (PKC) isoforms initiate a mitogen-activated protein kinase (MAPK) signaling cascade that regulates keratinocyte differentiation. However, assigning these functions has relied on treatment with pharmacologic inhibitors and/or manipulating kinase function using overexpression of wild-type or dominant-negative kinases. As these methods are not highly specific, an obligatory regulatory role for individual kinases has not been assigned. In this study, we use small interfering RNA knockdown to study the role of individual PKC isoforms as regulators of keratinocyte differentiation induced by the potent differentiating stimulus, 12-O-tetradecanoylphorbol-13-acetate (TPA). PKC-delta knockdown reduces TPA-activated involucrin promoter activity, nuclear activator protein-1 factor accumulation and binding to DNA, and cell morphology change. Knockdown of PKC downstream targets, including MEKK-1, MEK-6, MEK-3, or p38-delta, indicates that these kinases are required for these responses. Additional studies indicate that knockdown of PKC-eta inhibits TPA-dependent involucrin promoter activation. In contrast, knockdown of PKC-alpha (a classical PKC isoform) or PKC-epsilon (a novel isoform) does not inhibit these TPA-dependent responses. Further studies indicate that PKC-delta is required for calcium and green tea polyphenol-dependent regulation of end responses. These findings are informative as they suggest an essential role for selected PKC and MAPK cascade enzymes in mediating a range of end responses to a range of differentiation stimuli in keratinocytes.
Conflict of interest statement
The authors state no conflict of interest.
Figures
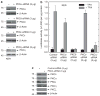
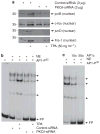
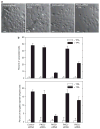



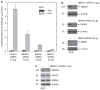
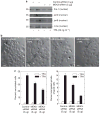
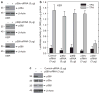

Comment in
-
Protein kinase C/mitogen-activated protein kinase signaling in keratinocyte differentiation control.J Invest Dermatol. 2010 Aug;130(8):1968-70. doi: 10.1038/jid.2010.194. J Invest Dermatol. 2010. PMID: 20631751
-
Findings of Research Misconduct.Fed Regist. 2024 Aug 15;89(158):66420-66422. Fed Regist. 2024. PMID: 39161428 Free PMC article. No abstract available.
References
-
- Acs P, Beheshti M, Szallasi Z, et al. Effect of a tyrosine 155 to phenylalanine mutation of protein kinase cdelta on the proliferative and tumorigenic properties of NIH 3T3 fibroblasts. Carcinogenesis. 2000;21:887–91. - PubMed
-
- Alt A, Ohba M, Li L, et al. Protein kinase Cdelta-mediated phosphorylation of alpha6beta4 is associated with reduced integrin localization to the hemidesmosome and decreased keratinocyte attachment. Cancer Res. 2001;61:4591–8. - PubMed
-
- Amar LS, Shabana A, Oboeuf M, et al. Desmosomes are regulated by protein kinase C in primary rat epithelial cells. Cell Adhes Commun. 1998;5:1–12. - PubMed
-
- Ashendel CL. The phorbol ester receptor: a phospholipid-regulated protein kinase. Biochim Biophys Acta. 1985;822:219–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

