Murine lupus susceptibility locus Sle1a requires the expression of two sub-loci to induce inflammatory T cells
- PMID: 20445563
- PMCID: PMC2958247
- DOI: 10.1038/gene.2010.23
Murine lupus susceptibility locus Sle1a requires the expression of two sub-loci to induce inflammatory T cells
Abstract
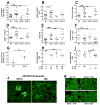
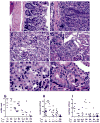
The NZM2410-derived Sle1a lupus susceptibility locus induces activated autoreactive CD4(+) T cells and reduces the number and function of Foxp3(+) regulatory T cells (Tregs). In this study, we first showed that Sle1a contributes to autoimmunity by increasing antinuclear antibody production when expressed on either NZB or NZW heterozygous genomes, and by enhancing the chronic graft versus host disease response indicating an expansion of the autoreactive B-cell pool. Screening two non-overlapping recombinants, the Sle1a.1 and Sle1a.2 intervals that cover the entire Sle1a locus, revealed that both Sle1a.1 and Sle1a.2 were necessary for the full Sle1a phenotype. Sle1a.1, and to a lesser extent Sle1a.2, significantly affected CD4(+) T-cell activation as well as Treg differentiation and function. Sle1a.2 also increased the production of autoreactive B cells. As the Sle1a.1 and Sle1a.2 intervals contain only 1 and 15 known genes, respectively, this study considerably reduces the number of candidate genes responsible for the production of autoreactive T cells. These results also show that the Sle1 locus is an excellent model for the genetic architecture of lupus, in which a major obligate phenotype results from the coexpression of multiple genetic variants with individual weak effects.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures





References
-
- Wakeland EK, Liu K, Graham RR, Behrens TW. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 2001;15:397–408. - PubMed
-
- Kono DH, Theofilopoulos AN. Genetics of SLE in mice. Springer Semin Immunopathol. 2006;28:83–96. - PubMed
-
- Morel L, Rudofsky UH, Longmate JA, Schiffenbauer J, Wakeland EK. Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity. 1994;1:219–229. - PubMed
-
- Waters ST, Fu SM, Gaskin F, Deshmukh US, Sung SSJ, Kannapell CC, et al. NZM2328: A new mouse model of systemic lupus erythematosus with unique genetic susceptibility loci. Clin Immunol. 2001;100:372–383. - PubMed
-
- Vyse TJ, Rozzo SJ, Drake CG, Izui S, Kotzin BL. Control of multiple autoantibodies linked with a lupus nephritis susceptibility locus in New Zealand Black mice. J Immunol. 1997;158:5566–5574. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

