Histological and immunohistochemical evaluation of autologous cultured bone marrow mesenchymal stem cells and bone marrow mononucleated cells in collagenase-induced tendinitis of equine superficial digital flexor tendon
- PMID: 20445779
- PMCID: PMC2859019
- DOI: 10.4061/2010/250978
Histological and immunohistochemical evaluation of autologous cultured bone marrow mesenchymal stem cells and bone marrow mononucleated cells in collagenase-induced tendinitis of equine superficial digital flexor tendon
Abstract
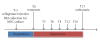
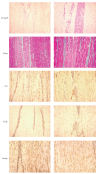
The aim of this study was to compare treatment with cultured bone marrow stromal cells (cBMSCs), bone marrow Mononucleated Cells (BMMNCs), and placebo to repair collagenase-induced tendinitis in horses. In six adult Standardbred horses, 4000 IU of collagenase were injected in the superficial digital flexor tendon (SDFT). Three weeks after collagenase treatment, an average of either 5.5 x 10(6) cBMSCs or 1.2 x 10(8) BMMNCs, fibrin glue, and saline solution was injected intralesionally in random order. In cBMSC- and BMMNCS-treated tendons, a high expression of cartilage oligomeric matrix protein (COMP) and type I collagen, but low levels of type III collagen were revealed by immunohistochemistry, with a normal longitudinally oriented fiber pattern. Placebo-treated tendons expressed very low quantities of COMP and type I collagen but large numbers of randomly oriented type III collagen fibers. Both cBMSC and BMMNCS grafts resulted in a qualitatively similar heling improvement of tendon extracellular matrix, in terms of the type I/III collagen ratio, fiber orientation, and COMP expression.
Figures




References
-
- Awad HA, Butler DL, Boivin GP, et al. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Engineering. 1999;5(3):267–277. - PubMed
-
- Ouyang HW, Goh JC, Thambyah A, et al. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit achilles tendon. Tissue Engineering. 2003;9(3):431–439. - PubMed
-
- Smith RK, Korda M, Blunn GW, et al. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Veterinary Journal. 2003;35(1):99–102. - PubMed
-
- Richardson LE, Dudhia J, Clegg PD, et al. Stem cells in veterinary medicine—attempts at regenerating equine tendon after injury. Trends in Biotechnology. 2007;25(9):409–416. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

