Roles of cysteines Cys115 and Cys201 in the assembly and thermostability of grouper betanodavirus particles
- PMID: 20446029
- PMCID: PMC2886913
- DOI: 10.1007/s11262-010-0488-1
Roles of cysteines Cys115 and Cys201 in the assembly and thermostability of grouper betanodavirus particles
Abstract
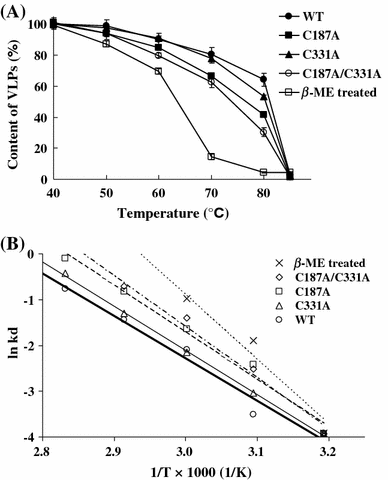
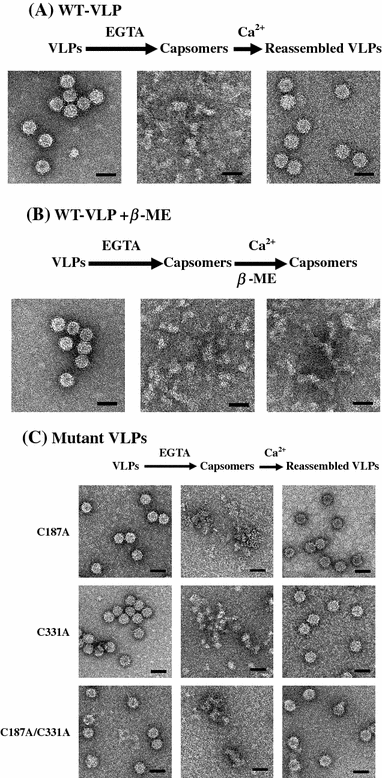
The virus-like particle (VLP) assembled from capsid subunits of the dragon grouper nervous necrosis virus (DGNNV) is very similar to its native T = 3 virion. In order to investigate the effects of four cysteine residues in the capsid polypeptide on the assembly/dissociation pathways of DGNNV virions, we recombinantly cloned mutant VLPs by mutating each cysteine to destroy the specific disulfide linkage as compared with thiol reduction to destroy all S-S bonds. The mutant VLPs of C187A and C331A mutations were similar to wild-type VLPs (WT-VLPs); hence, the effects of Cys187 and Cys331 on the particle formation and thermostability were presumably negligible. Electron microscopy showed that either C115A or C201A mutation disrupted de novo VLP formation significantly. As shown in micrographs and thermal decay curves, beta-mercaptoethanol-treated WT-VLPs remained intact, merely resulting in lower tolerance to thermal disruption than native WT-VLPs. This thiol reduction broke disulfide linkages inside the pre-fabricated VLPs, but it did not disrupt the appearance of icosahedrons. Small dissociated capsomers from EGTA-treated VLPs were able to reassemble back to icosahedrons in the presence of calcium ions, but additional treatment with beta-mercaptoethanol during EGTA dissociation resulted in inability of the capsomers to reassemble into the icosahedral form. These results indicated that Cys115 and Cys201 were essential for capsid formation of DGNNV icosahedron structure in de novo assembly and reassembly pathways, as well as for the thermal stability of pre-fabricated particles.
Figures

References
-
- Munday BL, Kwang J, Moody N. J. Fish Dis. 2002;25:127–142. doi: 10.1046/j.1365-2761.2002.00350.x. - DOI
-
- C.H. Wang, R.H. Cheng, C.S. Lin, The 108th General Meeting of American Society for Microbiology, Boston, MA, T-023 101 (2008)

