Approaches to N-methylwelwitindolinone C isothiocyanate: facile synthesis of the tetracyclic core
- PMID: 20446675
- PMCID: PMC2878383
- DOI: 10.1021/ol1006373
Approaches to N-methylwelwitindolinone C isothiocyanate: facile synthesis of the tetracyclic core
Abstract
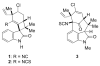
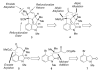
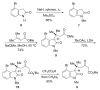
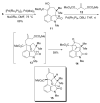
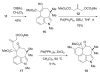
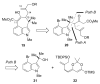
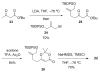
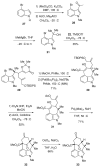
The synthesis of a functionalized, tetracyclic core of N-methylwelwitindolinone C isothiocyanate is reported. The approach features a convergent coupling between an indole iminium ion and a highly functionalized vinylogous silyl ketene acetal followed by an intramolecular palladium-catalyzed cyclization that proceeds via an enolate arylation.
Figures
References
-
- Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA. J. Am. Chem. Soc. 1994;116:9935–9942.
-
- Smith CD, Zilfou JT, Stratmann K, Patterson GML, Moore RE. Mol. Pharmacol. 1995;47:241–247. - PubMed
-
-
Reviews: Avendanó C, Menédez JC. Curr. Org. Synth. 2004;1:65–82. Menendez JC. Top. Heterocycl. Chem. 2007;11:63–101. Brown LE, Konopelski JP. Org. Prep. Proced. Int. 2008;40:411–445.
-
-
- Baran PS, Richter JM. J. Am. Chem. Soc. 2005;127:15394–15396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

