Molecular factors controlling photosynthetic light harvesting by carotenoids
- PMID: 20446691
- PMCID: PMC2923278
- DOI: 10.1021/ar100030m
Molecular factors controlling photosynthetic light harvesting by carotenoids
Abstract
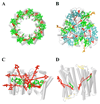
Carotenoids are naturally occurring pigments that absorb light in the spectral region in which the sun irradiates maximally. These molecules transfer this energy to chlorophylls, initiating the primary photochemical events of photosynthesis. Carotenoids also regulate the flow of energy within the photosynthetic apparatus and protect it from photoinduced damage caused by excess light absorption. To carry out these functions in nature, carotenoids are bound in discrete pigment-protein complexes in the proximity of chlorophylls. A few three-dimensional structures of these carotenoid complexes have been determined by X-ray crystallography. Thus, the stage is set for attempting to correlate the structural information with the spectroscopic properties of carotenoids to understand the molecular mechanism(s) of their function in photosynthetic systems. In this Account, we summarize current spectroscopic data describing the excited state energies and ultrafast dynamics of purified carotenoids in solution and bound in light-harvesting complexes from purple bacteria, marine algae, and green plants. Many of these complexes can be modified using mutagenesis or pigment exchange which facilitates the elucidation of correlations between structure and function. We describe the structural and electronic factors controlling the function of carotenoids as energy donors. We also discuss unresolved issues related to the nature of spectroscopically dark excited states, which could play a role in light harvesting. To illustrate the interplay between structural determinations and spectroscopic investigations that exemplifies work in the field, we describe the spectroscopic properties of four light-harvesting complexes whose structures have been determined to atomic resolution. The first, the LH2 complex from the purple bacterium Rhodopseudomonas acidophila, contains the carotenoid rhodopin glucoside. The second is the LHCII trimeric complex from higher plants which uses the carotenoids lutein, neoxanthin, and violaxanthin to transfer energy to chlorophyll. The third, the peridinin-chlorophyll-protein (PCP) from the dinoflagellate Amphidinium carterae, is the only known complex in which the bound carotenoid (peridinin) pigments outnumber the chlorophylls. The last is xanthorhodopsin from the eubacterium Salinibacter ruber. This complex contains the carotenoid salinixanthin, which transfers energy to a retinal chromophore. The carotenoids in these pigment-protein complexes transfer energy with high efficiency by optimizing both the distance and orientation of the carotenoid donor and chlorophyll acceptor molecules. Importantly, the versatility and robustness of carotenoids in these light-harvesting pigment-protein complexes have led to their incorporation in the design and synthesis of nanoscale antenna systems. In these bioinspired systems, researchers are seeking to improve the light capture and use of energy from the solar emission spectrum.
Figures


References
-
- Isler O, editor. Carotenoids. Basel: 1971.
-
- Hudson BS, Kohler BE. A low-lying weak transition in polyene α,ω-diphenyloctatetraene. Chem. Phys. Lett. 1972;14:299–304.
-
- Polívka T, Sundström V. Ultrafast dynamics of carotenoid excited states - From solution to natural and artificial systems. Chem. Rev. 2004;104:2021–2071. - PubMed
-
- Polívka T, Sundström V. Dark excited states of carotenoids: Consensus and controversy. Chem. Phys. Lett. 2009;477:1–11.
-
- Krueger BP, Scholes GD, Jimenez R, Fleming GR. Electronic excitation transfer from carotenoid to bacteriochlorophyll in the purple bacterium Rhodopseudomonas acidophila. J. Phys. Chem. B. 1998;102:2284–2292.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

