Pyrimidine ribonucleotides with enhanced selectivity as P2Y(6) receptor agonists: novel 4-alkyloxyimino, (S)-methanocarba, and 5'-triphosphate gamma-ester modifications
- PMID: 20446735
- PMCID: PMC2935147
- DOI: 10.1021/jm100287t
Pyrimidine ribonucleotides with enhanced selectivity as P2Y(6) receptor agonists: novel 4-alkyloxyimino, (S)-methanocarba, and 5'-triphosphate gamma-ester modifications
Abstract
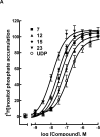
The P2Y(6) receptor is a cytoprotective G-protein-coupled receptor (GPCR) activated by UDP (EC(50) = 0.30 microM). We compared and combined modifications to enhance P2Y(6) receptor agonist selectivity, including ribose ring constraint, 5-iodo and 4-alkyloxyimino modifications, and phosphate modifications such as alpha,beta-methylene and extension of the terminal phosphate group into gamma-esters of UTP analogues. The conformationally constrained (S)-methanocarba-UDP is a full agonist (EC(50) = 0.042 microM). 4-Methoxyimino modification of pyrimidine enhanced P2Y(6), preserved P2Y(2) and P2Y(4), and abolished P2Y(14) receptor potency, in the appropriate nucleotide. N(4)-Benzyloxy-CDP (15, MRS2964) and N(4)-methoxy-Cp(3)U (23, MRS2957) were potent, selective P2Y(6) receptor agonists (EC(50) of 0.026 and 0.012 microM, respectively). A hydrophobic binding region near the nucleobase was explored with receptor modeling and docking. UTP-gamma-aryl and cycloalkyl phosphoesters displayed only intermediate P2Y(6) receptor potency but had enhanced stability in acid and cell membranes. UTP-glucose was inactive, but its (S)-methanocarba analogue and N(4)-methoxycytidine 5'-triphospho-gamma-[1]glucose were active (EC(50) of 2.47 and 0.18 microM, respectively). Thus, the potency, selectivity, and stability of pyrimidine nucleotides as P2Y(6) receptor agonists may be enhanced by modest structural changes.
Figures







Similar articles
-
Adenine nucleotide analogues locked in a Northern methanocarba conformation: enhanced stability and potency as P2Y(1) receptor agonists.J Med Chem. 2002 May 9;45(10):2090-100. doi: 10.1021/jm010538v. J Med Chem. 2002. PMID: 11985476 Free PMC article.
-
Pyrimidine nucleotides with 4-alkyloxyimino and terminal tetraphosphate δ-ester modifications as selective agonists of the P2Y(4) receptor.J Med Chem. 2011 Jun 23;54(12):4018-33. doi: 10.1021/jm101591j. Epub 2011 May 20. J Med Chem. 2011. PMID: 21528910 Free PMC article.
-
Methanocarba modification of uracil and adenine nucleotides: high potency of Northern ring conformation at P2Y1, P2Y2, P2Y4, and P2Y11 but not P2Y6 receptors.J Med Chem. 2002 Jan 3;45(1):208-18. doi: 10.1021/jm010369e. J Med Chem. 2002. PMID: 11754592 Free PMC article.
-
P2-pyrimidinergic receptors and their ligands.Curr Pharm Des. 2002;8(26):2353-69. doi: 10.2174/1381612023392937. Curr Pharm Des. 2002. PMID: 12369950 Review.
-
Development of selective high affinity antagonists, agonists, and radioligands for the P2Y1 receptor.Comb Chem High Throughput Screen. 2008 Jul;11(6):410-9. doi: 10.2174/138620708784911474. Comb Chem High Throughput Screen. 2008. PMID: 18673269 Free PMC article. Review.
Cited by
-
Design, synthesis, pharmacological characterization of a fluorescent agonist of the P2Y₁₄ receptor.Bioorg Med Chem Lett. 2015 Nov 1;25(21):4733-4739. doi: 10.1016/j.bmcl.2015.08.021. Epub 2015 Aug 10. Bioorg Med Chem Lett. 2015. PMID: 26303895 Free PMC article.
-
South (S)- and North (N)-Methanocarba-7-Deazaadenosine Analogues as Inhibitors of Human Adenosine Kinase.J Med Chem. 2016 Jul 28;59(14):6860-77. doi: 10.1021/acs.jmedchem.6b00689. Epub 2016 Jul 13. J Med Chem. 2016. PMID: 27410258 Free PMC article.
-
Pharmacological characterization of P2Y receptor subtypes - an update.Purinergic Signal. 2024 Apr;20(2):99-108. doi: 10.1007/s11302-023-09963-w. Epub 2023 Sep 12. Purinergic Signal. 2024. PMID: 37697211 Free PMC article. Review.
-
Methanocarba ring as a ribose modification in ligands of G protein-coupled purine and pyrimidine receptors: synthetic approaches.Medchemcomm. 2013 Dec 17;2013(4):619-630. doi: 10.1039/C2MD20348K. Medchemcomm. 2013. PMID: 26161251 Free PMC article.
-
Pyrimidine Nucleotides Containing a (S)-Methanocarba Ring as P2Y6 Receptor Agonists.Medchemcomm. 2017 Oct 1;8(10):1897-1908. doi: 10.1039/C7MD00397H. Epub 2017 Sep 6. Medchemcomm. 2017. PMID: 29423136 Free PMC article.
References
-
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006;58:281–341. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

