Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/β-catenin signaling
- PMID: 20446815
- PMCID: PMC3128758
- DOI: 10.1089/scd.2010.0013
Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/β-catenin signaling
Abstract
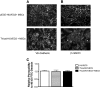
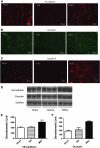
The barrier formed by endothelial cells (ECs) plays an important role in tissue homeostasis by restricting passage of circulating molecules and inflammatory cells. Disruption of the endothelial barrier in pathologic conditions often leads to uncontrolled inflammation and tissue damage. An important component of this barrier is adherens junctions, which restrict paracellular permeability. The transmembrane protein vascular endothelial (VE)-cadherin and its cytoplasmic binding partner β-catenin are major components of functional adherens junctions. We show that mesenchymal stem cells (MSCs) significantly reduce endothelial permeability in cocultured human umbilical vascular endothelial cells (HUVECs) and following exposure to vascular endothelial growth factor, a potent barrier permeability-enhancing agent. When grown in cocultures with HUVECs, MSCs increased VE-cadherin levels and enhanced recruitment of both VE-cadherin and β-catenin to the plasma membrane. Enhanced membrane localization of β-catenin was associated with a decrease in β-catenin-driven gene transcription. Disruption of the VE-cadherin/β-catenin interaction by overexpressing a truncated VE-cadherin lacking the β-catenin interacting domain blocked the permeability-stabilizing effect of MSCs. Interestingly, a conditioned medium from HUVEC-MSC cocultures, but not from HUVEC or MSC cells cultured alone, significantly reduced endothelial permeability. In addition, intravenous administration of MSCs to brain-injured rodents reduced injury-induced enhanced blood-brain barrier permeability. Similar to the effect on in vitro cultures, this stabilizing effect on blood-brain barrier function was associated with increased expression of VE-cadherin. Taken together, these results identify a putative mechanism by which MSCs can modulate vascular EC permeability. Further, our results suggest that the mediator(s) of these vascular protective effects is a secreted factor(s) released as a result of direct MSC-EC interaction.
Figures





References
-
- Dejana E. Orsenigo F. Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. - PubMed
-
- Pittenger MF. Mesenchymal stem cells from adult bone marrow. Methods Mol Biol. 2008;449:27–44. - PubMed
-
- Qian H. Yang H. Xu W, et al. Bone marrow mesenchymal stem cells ameliorate rat acute renal failure by differentiation into renal tubular epithelial-like cells. Int J Mol Med. 2008;22:325–332. - PubMed
-
- Le Blanc K. Rasmusson I. Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

