Eosinophils in health and disease: the LIAR hypothesis
- PMID: 20447076
- PMCID: PMC2951476
- DOI: 10.1111/j.1365-2222.2010.03484.x
Eosinophils in health and disease: the LIAR hypothesis
Abstract
Discussions of eosinophils are often descriptions of end-stage effector cells with destructive capabilities mediated predominantly by released cytotoxic cationic granule proteins. Moreover, eosinophils in the medical literature are invariably associated with the pathologies linked with helminth infections or allergic diseases such as asthma. This has led to an almost fatalist view of eosinophil effector functions and associated therapeutic strategies targeting these cells that would make even William of Ockham proud - eosinophil effector functions have physiological consequences that increase patient morbidity/mortality and 'the only good eosinophils are dead eosinophils'. Unfortunately, the strengths of dogmas are also their greatest weaknesses. Namely, while the repetitive proclamation of dogmatic concepts by authoritative sources (i.e. reviews, meeting proceedings, textbooks, etc.) builds consensus within the medical community and lower the entropies surrounding difficult issues, they often ignore not easily explained details and place diminished importance on alternative hypotheses. The goal of this perspective is twofold: (i) we will review recent observations regarding eosinophils and their activities as well as reinterpret earlier data as part of the synthesis of a new paradigm. In this paradigm, we hypothesize that eosinophils accumulate at unique sites in response to cell turnover or in response to local stem cell activity(ies). We further suggest that this accumulation is part of one or more mechanisms regulating tissue homeostasis. Specifically, instead of immune cells exclusively mediating innate host defence, we suggest that accumulating tissue eosinophils are actually regulators of Local Immunity And/or Remodeling/Repair in both health and disease - the LIAR hypothesis; (ii) we want to be inflammatory (pun intended!) and challenge the currently common perspective of eosinophils as destructive end-stage effector cells. Our hope is to create more questions than we answer and provoke everyone to spend countless hours simply to prove us wrong!
Figures
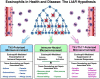
 ) recruitment occurs in response to the release of one or more small molecule mediators of inflammation (e.g., DAMPs) released from localized bursts of cell death (
) recruitment occurs in response to the release of one or more small molecule mediators of inflammation (e.g., DAMPs) released from localized bursts of cell death ( ). In the presence of additional eosinophil agonist growth (e.g., IL-5) and survival (e.g., GM-CSF) factors derived from concomitant cell proliferation and/or stem cell activation (
). In the presence of additional eosinophil agonist growth (e.g., IL-5) and survival (e.g., GM-CSF) factors derived from concomitant cell proliferation and/or stem cell activation ( ), these granulocytes accumulate and establish a local steady-state population. The tissue immune microenvironment subsequently dictates the downstream immune consequences mediated by eosinophil effector functions, leading either to exacerbations of local immune responses (Th2-Polarized Microenvironment), suppression of these site-specific immune responses (Th1/Th17-Polarized Microenvironment), or essentially little to no modulations of local immune responses (Immune-Neutral Microenvironment). In turn, these immune responses modulate the levels of tissue remodeling and/or tissue repair that is also characteristic of eosinophil-mediated effector functions. Thus, the immune microenvironment present upon eosinophil recruitment is a significant situational cue which drives the predominance of specific eosinophil activities. More importantly, this eosinophil-mediated
), these granulocytes accumulate and establish a local steady-state population. The tissue immune microenvironment subsequently dictates the downstream immune consequences mediated by eosinophil effector functions, leading either to exacerbations of local immune responses (Th2-Polarized Microenvironment), suppression of these site-specific immune responses (Th1/Th17-Polarized Microenvironment), or essentially little to no modulations of local immune responses (Immune-Neutral Microenvironment). In turn, these immune responses modulate the levels of tissue remodeling and/or tissue repair that is also characteristic of eosinophil-mediated effector functions. Thus, the immune microenvironment present upon eosinophil recruitment is a significant situational cue which drives the predominance of specific eosinophil activities. More importantly, this eosinophil-mediated 
References
-
- Austen KF. Homeostasis of effector systems which can also be recruited for immunologic reactions. J Immunol. 1978;121:793–805. Review. - PubMed
-
- Smith H, Cook RM. Immunopharmacology of Eosinophils. In: Page CF, editor. The Handbook of Immunopharmacology. 1st ed. Academic Press, Harcourt Brace Jovanovich; London: 1993. p. 250.
-
- Taliaferro WH, Sarles MP. The cellular reactions in the skin, lungs, and intestine of normal and immune rats after infection with Nippostrongylus muris. J Infect Dis. 1939;64:157–192.
-
- Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113:30–37. - PubMed
-
- Bruschi F, Korenaga M, Watanabe N. Eosinophils and Trichinella infection: toxic for the parasite and the host? Trends Parasitol. 2008;24:462–467. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

