Activation of geminivirus V-sense promoters in roots is restricted to nematode feeding sites
- PMID: 20447288
- PMCID: PMC6640434
- DOI: 10.1111/j.1364-3703.2010.00611.x
Activation of geminivirus V-sense promoters in roots is restricted to nematode feeding sites
Abstract
Obligate sedentary endoparasitic nematodes, such as the root-knot and cyst nematodes, elicit the differentiation of specialized nematode nurse or feeding cells [nematode feeding sites (NFS), giant cells and syncytia, respectively]. During NFS differentiation, marked changes in cell cycle progression occur, partly similar to those induced by some geminiviruses. In this work, we describe the activation of V-sense promoters from the Maize streak virus (MSV) and Wheat dwarf virus (WDV) in NFS formed by root-knot and cyst nematodes. Both promoters were transiently active in microinjection experiments. In tobacco and Arabidopsis transgenic lines carrying promoter-beta-glucuronidase fusions, the MSV V-sense promoter was activated in the vascular tissues of aerial plant parts, primarily leaf and cotyledon phloem tissue and some floral structures. Interestingly, in roots, promoter activation was restricted to syncytia and giant cells tested with four different nematode populations, but undetectable in the rest of the root system. As the activity of the promoter in transgenic rootstocks should be restricted to NFS only, the MSV promoter may have utility in engineering grafted crops for nematode control. Therefore, this study represents a step in the provision of some of the much needed additional data on promoters with restricted activation in NFS useful in biotechnological nematode control strategies.
Figures
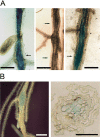

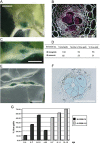
Similar articles
-
The role of callose deposition along plasmodesmata in nematode feeding sites.Mol Plant Microbe Interact. 2010 May;23(5):549-57. doi: 10.1094/MPMI-23-5-0549. Mol Plant Microbe Interact. 2010. PMID: 20367463
-
Distinct heat-shock element arrangements that mediate the heat shock, but not the late-embryogenesis induction of small heat-shock proteins, correlate with promoter activation in root-knot nematode feeding cells.Plant Mol Biol. 2008 Jan;66(1-2):151-64. doi: 10.1007/s11103-007-9259-3. Epub 2007 Nov 28. Plant Mol Biol. 2008. PMID: 18046507
-
Analysis of nematode-responsive promoters in sugar beet hairy roots.Meded Rijksuniv Gent Fak Landbouwkd Toegep Biol Wet. 2001;66(2b):591-8. Meded Rijksuniv Gent Fak Landbouwkd Toegep Biol Wet. 2001. PMID: 12425082
-
Cell cycle activation by plant parasitic nematodes.Plant Mol Biol. 2000 Aug;43(5-6):747-61. doi: 10.1023/a:1006367126077. Plant Mol Biol. 2000. PMID: 11089874 Review.
-
Nematode feeding sites: unique organs in plant roots.Planta. 2013 Nov;238(5):807-18. doi: 10.1007/s00425-013-1923-z. Epub 2013 Jul 4. Planta. 2013. PMID: 23824525 Review.
Cited by
-
Piriformospora indica promotes cucumber tolerance against Root-knot nematode by modulating photosynthesis and innate responsive genes.Saudi J Biol Sci. 2020 Jan;27(1):279-287. doi: 10.1016/j.sjbs.2019.09.007. Epub 2019 Sep 11. Saudi J Biol Sci. 2020. PMID: 31889848 Free PMC article.
-
Long-Term In Vitro System for Maintenance and Amplification of Root-Knot Nematodes in Cucumis sativus Roots.Front Plant Sci. 2016 Feb 22;7:124. doi: 10.3389/fpls.2016.00124. eCollection 2016. Front Plant Sci. 2016. PMID: 26941745 Free PMC article.
References
-
- De Almeida Engler, J. , De Vleesschauwer, V. , Burssens, S. , Celenza, J.L. Jr , Inze, D. , Van Montagu, M. , Engler, G. and Gheysen, G. (1999) Molecular markers and cell cycle inhibitors show the importance of cell cycle progression in nematode‐induced galls and syncytia. Plant Cell, 11, 793–808. - PMC - PubMed
-
- Ascencio‐Ibanez, J.T. , Sozzani, R. , Lee, T.J. , Chu, T.M. , Wolfinger, R.D. , Cella, R. and Hanley‐Bowdoin, L. (2008) Global analysis of Arabidopsis gene expression uncovers a complex array of changes impacting pathogen response and cell cycle during geminivirus infection. Plant Physiol. 148, 436–454. - PMC - PubMed
-
- Barcala, M. , Garcia, A. , Cubas, P. , Almoguera, C. , Jordano, J. , Fenoll, C. and Escobar, C. (2008) Distinct heat‐shock element arrangements that mediate the heat shock, but not the late‐embryogenesis induction of small heat‐shock proteins, correlate with promoter activation in root‐knot nematode feeding cells. Plant Mol. Biol. 66, 151–164. - PubMed
-
- Bockenhoff, A. and Grundler, F.M. (1994) Studies on the nutrient uptake by the beet cyst nematode Heterodera schachtii by in situ microinjection of fluorescent probes into the feeding structure in Arabidopsis thaliana . Parasitology, 109, 249–254.
-
- Caillaud, M.C. , Dubreuil, G. , Quentin, M. , Perfus‐Barbeoch, L. , Lecomte, P. , De Almeida Engler, J. , Abad, P. , Rosso, M.N. and Favery, B. (2008) Root‐knot nematodes manipulate plant cell functions during a compatible interaction. J. Plant Physiol. 165, 104–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

