Transport and equilibrium uptake of a peptide inhibitor of PACE4 into articular cartilage is dominated by electrostatic interactions
- PMID: 20447377
- PMCID: PMC2885539
- DOI: 10.1016/j.abb.2010.04.019
Transport and equilibrium uptake of a peptide inhibitor of PACE4 into articular cartilage is dominated by electrostatic interactions
Abstract
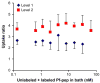
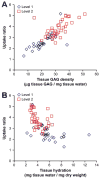
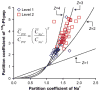
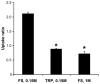
The availability of therapeutic molecules to targets within cartilage depends on transport through the avascular matrix. We studied equilibrium partitioning and non-equilibrium transport into cartilage of Pf-pep, a 760 Da positively charged peptide inhibitor of the proprotein convertase PACE4. Competitive binding measurements revealed negligible binding of Pf-pep to sites within cartilage. Uptake of Pf-pep depended on glycosaminoglycan charge density, and was consistent with predictions of Donnan equilibrium given the known charge of Pf-pep. In separate transport experiments, the diffusivity of Pf-pep in cartilage was measured to be approximately 1 x 10(-6) cm(2)/s, close to other similarly-sized non-binding solutes. These results suggest that small positively charged therapeutics will have a higher concentration within cartilage than in the surrounding synovial fluid, a desired property for local delivery; however, such therapeutics may rapidly diffuse out of cartilage unless there is additional specific binding to intra-tissue substrates that can maintain enhanced intra-tissue concentration for local delivery.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Cartilage-targeting drug delivery: can electrostatic interactions help?Nat Rev Rheumatol. 2017 Mar;13(3):183-193. doi: 10.1038/nrrheum.2016.210. Epub 2017 Feb 9. Nat Rev Rheumatol. 2017. PMID: 28202920 Review.
-
Cartilage penetrating cationic peptide carriers for applications in drug delivery to avascular negatively charged tissues.Acta Biomater. 2019 Jul 15;93:258-269. doi: 10.1016/j.actbio.2018.12.004. Epub 2018 Dec 6. Acta Biomater. 2019. PMID: 30529083 Free PMC article.
-
Electrostatic interactions enable rapid penetration, enhanced uptake and retention of intra-articular injected avidin in rat knee joints.J Orthop Res. 2014 Aug;32(8):1044-51. doi: 10.1002/jor.22630. Epub 2014 Apr 21. J Orthop Res. 2014. PMID: 24753019
-
Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis.Biomaterials. 2014 Jan;35(1):538-49. doi: 10.1016/j.biomaterials.2013.09.091. Epub 2013 Oct 10. Biomaterials. 2014. PMID: 24120044 Free PMC article.
-
How can 50 years of solute transport data in articular cartilage inform the design of arthritis therapeutics?Osteoarthritis Cartilage. 2018 Nov;26(11):1438-1446. doi: 10.1016/j.joca.2018.07.006. Epub 2018 Jul 24. Osteoarthritis Cartilage. 2018. PMID: 30053617 Review.
Cited by
-
Contrast-enhanced CT with a high-affinity cationic contrast agent for imaging ex vivo bovine, intact ex vivo rabbit, and in vivo rabbit cartilage.Radiology. 2013 Jan;266(1):141-50. doi: 10.1148/radiol.12112246. Epub 2012 Nov 28. Radiology. 2013. PMID: 23192774 Free PMC article.
-
Green fluorescent proteins engineered for cartilage-targeted drug delivery: Insights for transport into highly charged avascular tissues.Biomaterials. 2018 Nov;183:218-233. doi: 10.1016/j.biomaterials.2018.08.050. Epub 2018 Aug 25. Biomaterials. 2018. PMID: 30173104 Free PMC article.
-
Multi-Trait Genome-Wide Association Study of Atherosclerosis Detects Novel Pleiotropic Loci.Front Genet. 2022 Feb 2;12:787545. doi: 10.3389/fgene.2021.787545. eCollection 2021. Front Genet. 2022. PMID: 35186008 Free PMC article.
-
Cartilage-targeting drug delivery: can electrostatic interactions help?Nat Rev Rheumatol. 2017 Mar;13(3):183-193. doi: 10.1038/nrrheum.2016.210. Epub 2017 Feb 9. Nat Rev Rheumatol. 2017. PMID: 28202920 Review.
-
On the cutting edge of proprotein convertase pharmacology: from molecular concepts to clinical applications.Biomol Concepts. 2011 Oct 1;2(5):421-438. doi: 10.1515/bmc.2011.034. Biomol Concepts. 2011. PMID: 22308173 Free PMC article.
References
-
- Malfait AM, Arner EC, Song RH, Alston JT, Markosyan S, Staten N, Yang Z, Griggs DW, Tortorella MD. Proprotein convertase activation of aggrecanases in cartilage in situ. Arch Biochem Biophys. 2008;478:43–51. - PubMed
-
- Tortorella MD, Burn TC, Pratta MA, Abbaszade I, Hollis JM, Liu R, Rosenfeld SA, Copeland RA, Decicco CP, Wynn R, Rockwell A, Yang F, Duke JL, Solomon K, George H, Bruckner R, Nagase H, Itoh Y, Ellis DM, Ross H, Wiswall BH, Murphy K, Hillman MC, Jr, Hollis GF, Newton RC, Magolda RL, Trzaskos JM, Arner EC. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science. 1999;284:1664–6. - PubMed
-
- Abbaszade I, Liu RQ, Yang F, Rosenfeld SA, Ross OH, Link JR, Ellis DM, Tortorella MD, Pratta MA, Hollis JM, Wynn R, Duke JL, George HJ, Hillman MC, Jr, Murphy K, Wiswall BH, Copeland RA, Decicco CP, Bruckner R, Nagase H, Itoh Y, Newton RC, Magolda RL, Trzaskos JM, Hollis GF, Arner EC, Burn TC. Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem. 1999;274:23443–50. - PubMed
-
- Tortorella MD, Arner EC, Hills R, Gormley J, Fok K, Pegg L, Munie G, Malfait AM. ADAMTS-4 (aggrecanase-1): N-terminal activation mechanisms. Arch Biochem Biophys. 2005;444:34–44. - PubMed
-
- Wang P, Tortorella M, England K, Malfait AM, Thomas G, Arner EC, Pei D. Proprotein convertase furin interacts with and cleaves pro-ADAMTS4 (Aggrecanase-1) in the trans-Golgi network. J Biol Chem. 2004;279:15434–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

