Identification and pre-clinical testing of a reversible cathepsin protease inhibitor reveals anti-tumor efficacy in a pancreatic cancer model
- PMID: 20447439
- PMCID: PMC3814225
- DOI: 10.1016/j.biochi.2010.04.023
Identification and pre-clinical testing of a reversible cathepsin protease inhibitor reveals anti-tumor efficacy in a pancreatic cancer model
Abstract
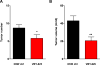
Proteolytic activity is required for several key processes in cancer development and progression, including tumor growth, invasion and metastasis. Accordingly, high levels of protease expression and activity have been found to correlate with malignant progression and poor patient prognosis in a wide variety of human cancers. Members of the papain family of cysteine cathepsins are among the protease classes that have been functionally implicated in cancer. Therefore, the discovery of effective cathepsin inhibitors has considerable potential for anti-cancer therapy. In this study we describe the identification of a novel, reversible cathepsin inhibitor, VBY-825, which has high potency against cathepsins B, L, S and V. VBY-825 was tested in a pre-clinical model of pancreatic islet cancer and found to significantly decrease tumor burden and tumor number. Thus, the identification of VBY-825 as a new and effective anti-tumor drug encourages the therapeutic application of cathepsin inhibitors in cancer.
Copyright © 2010 Elsevier Masson SAS. All rights reserved.
Figures



Similar articles
-
Inhibition of cysteine cathepsin protease activity enhances chemotherapy regimens by decreasing tumor growth and invasiveness in a mouse model of multistage cancer.Cancer Res. 2007 Aug 1;67(15):7378-85. doi: 10.1158/0008-5472.CAN-07-0602. Cancer Res. 2007. PMID: 17671208
-
ASPER-29 suppresses the metastasis of pancreatic cancer cells by dual inhibition of cathepsin-L and cathepsin-S.Chem Biol Interact. 2022 Feb 1;353:109811. doi: 10.1016/j.cbi.2022.109811. Epub 2022 Jan 8. Chem Biol Interact. 2022. PMID: 35016848
-
Distinct roles for cysteine cathepsin genes in multistage tumorigenesis.Genes Dev. 2006 Mar 1;20(5):543-56. doi: 10.1101/gad.1407406. Epub 2006 Feb 15. Genes Dev. 2006. PMID: 16481467 Free PMC article.
-
Novel Opportunities for Cathepsin S Inhibitors in Cancer Immunotherapy by Nanocarrier-Mediated Delivery.Cells. 2020 Sep 2;9(9):2021. doi: 10.3390/cells9092021. Cells. 2020. PMID: 32887380 Free PMC article. Review.
-
Cathepsins and pancreatic cancer: the 2012 update.Pancreatology. 2012 Sep-Oct;12(5):395-401. doi: 10.1016/j.pan.2012.07.011. Epub 2012 Jul 20. Pancreatology. 2012. PMID: 23127526 Review.
Cited by
-
A novel cysteine cathepsin inhibitor yields macrophage cell death and mammary tumor regression.Oncogene. 2015 Dec 10;34(50):6066-78. doi: 10.1038/onc.2015.51. Epub 2015 Mar 23. Oncogene. 2015. PMID: 25798843
-
Cathepsin K inhibition-induced mitochondrial ROS enhances sensitivity of cancer cells to anti-cancer drugs through USP27x-mediated Bim protein stabilization.Redox Biol. 2020 Feb;30:101422. doi: 10.1016/j.redox.2019.101422. Epub 2019 Dec 31. Redox Biol. 2020. PMID: 31901727 Free PMC article.
-
Detection of protease activities by flash chronopotentiometry using a reversible polycation-sensitive polymeric membrane electrode.Anal Biochem. 2011 Sep 1;416(1):67-73. doi: 10.1016/j.ab.2011.04.036. Epub 2011 Apr 29. Anal Biochem. 2011. PMID: 21601559 Free PMC article.
-
A novel in vitro system of supported planar endosomal membranes (SPEMs) reveals an enhancing role for cathepsin B in the final stage of Ebola virus fusion and entry.Microbiol Spectr. 2023 Sep 20;11(5):e0190823. doi: 10.1128/spectrum.01908-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 37728342 Free PMC article.
-
Cathepsins in digestive cancers.Oncotarget. 2017 Jun 20;8(25):41690-41700. doi: 10.18632/oncotarget.16677. Oncotarget. 2017. PMID: 28402938 Free PMC article. Review.
References
-
- Rowe RG, Weiss SJ. Navigating ECM barriers at the invasive front: the cancer cell-stroma interface. Annu Rev Cell Dev Biol. 2009;25:567–595. - PubMed
-
- Mason SD, Joyce JA. In: Proteolytic cascades in invasion and metastasis, Cancer Metastasis: Biologic Basis and Therapeutics. Welsh DR, Lyden DC, Psaila B, editors. Cambridge University Press; 2010. in press.
-
- Overall CM, Kleifeld O. Tumour microenvironment-opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. - PubMed
-
- Palermo C, Joyce JA. Cysteine cathepsin proteases as pharmacological targets in cancer. Trends Pharmacol Sci. 2008;29:22–28. - PubMed
-
- Ulisse S, Baldini E, Sorrenti S, D'Armiento M. The urokinase plasminogen activator system: a target for anti-cancer therapy. Curr Cancer Drug Targets. 2009;9:32–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

