In vivo studies of the SERT-selective [18F]FPBM and VMAT2-selective [18F]AV-133 radiotracers in a rat model of Parkinson's disease
- PMID: 20447560
- PMCID: PMC2909692
- DOI: 10.1016/j.nucmedbio.2010.01.006
In vivo studies of the SERT-selective [18F]FPBM and VMAT2-selective [18F]AV-133 radiotracers in a rat model of Parkinson's disease
Abstract
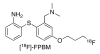
Introduction: The utility of [(18)F]FPBM [2-(2'-((dimethylamino)methyl)-4'-(3-[(18)F]-fluoropropoxy)phenylthio)benzenamine], a selective serotonin transporter (SERT) tracer, and [(18)F]AV-133 [(+)-2-Hydroxy-3-isobutyl-9-(3-fluoropropoxy)-10-methoxy-1,2,3,4,6,7-hexahydro-11bH-benzo[a]quinolizine], a selective vesicular monoamine transporter 2 (VMAT2) tracer, were tested in the 6-hydroxydopamine (6-OHDA) unilateral lesioned rat model.
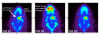
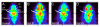
Methods: Positron emission tomography (PET) imaging of three 6-OHDA unilateral lesioned male Sprague Dawley rats (Rats 1-3) were performed with [(18)F]FPBM and [(18)F]AV-133 to examine whether changes in SERT and VMAT2 binding, respectively, could be detected in the brain. The brains of the three rats were then removed and examined by in vitro autoradiography with [(18)F]FPBM and the dopamine transporter ligand, [(125)I]IPT [N-(3'-[(125)I]-iodopropen-2'-yl)-2-beta-carbomethoxy-3-beta-(4-chloro phenyl) tropane, for confirmation. Biodistribution of [(18)F]FPBM in a separate group of p-chloroamphetamine (PCA) treated rats were also performed.
Results: PET image analysis showed varying levels of SERT binding reduction (Rat 1=-11%, Rat 2=-4%, Rat 3=-43%; n=2) and a clear and definitive loss of VMAT2 binding (Rat 1=-87%, Rat 2=-72%, and Rat 3=-91%; n=1) in the left striatum when compared to the right (non-lesioned side) striatum. The results from PET imaging were corroborated with quantitative in vitro autoradiography. Rats treated with a selective serotonin toxin (p-chloroamphetamine) showed a significant reduction of [(18)F]FPBM uptake in the cortex and hypothalamus regions of the brain.
Conclusion: The preliminary data suggest that [(18)F]FPBM and [(18)F]AV-133 may be useful for the examination of serotonergic and dopaminergic neuron integrity, respectively, in the living brain.
(c) 2010 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
An improved preparation of [18F]FPBM: a potential serotonin transporter (SERT) imaging agent.Nucl Med Biol. 2013 Nov;40(8):974-9. doi: 10.1016/j.nucmedbio.2013.08.002. Epub 2013 Sep 10. Nucl Med Biol. 2013. PMID: 24035549
-
Imaging of VMAT2 binding sites in the brain by (18)F-AV-133: the effect of a pseudo-carrier.Nucl Med Biol. 2012 Oct;39(7):897-904. doi: 10.1016/j.nucmedbio.2012.05.002. Epub 2012 Jun 28. Nucl Med Biol. 2012. PMID: 22749185
-
One-step preparation of [(18)F]FPBM for PET imaging of serotonin transporter (SERT) in the brain.Nucl Med Biol. 2016 Aug;43(8):470-7. doi: 10.1016/j.nucmedbio.2016.04.003. Epub 2016 Apr 19. Nucl Med Biol. 2016. PMID: 27236282
-
(+)-2-Hydroxy-3-isobutyl-9-(3-[18F]fluoropropoxy)-10-methoxy-1,2,3,4,6,7-hexahydro-11bH-benzo[a]quinolizine.2007 Feb 12 [updated 2010 Oct 14]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2007 Feb 12 [updated 2010 Oct 14]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641662 Free Books & Documents. Review.
-
2-Hydroxy-3-isobutyl-9-[11C]methoxy-10-methoxy-1,2,3,4,6,7,-hexahydro-11bH-bezo[α]-quinolizine.2006 Mar 17 [updated 2010 Oct 27]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2006 Mar 17 [updated 2010 Oct 27]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641794 Free Books & Documents. Review.
Cited by
-
PET imaging a MPTP-induced mouse model of Parkinson's disease using the fluoropropyl-dihydrotetrabenazine analog [18F]-DTBZ (AV-133).PLoS One. 2012;7(6):e39041. doi: 10.1371/journal.pone.0039041. Epub 2012 Jun 18. PLoS One. 2012. PMID: 22723923 Free PMC article.
-
Striatal serotonin transporter gain-of-function in L-DOPA-treated, hemi-parkinsonian rats.Brain Res. 2023 Jul 15;1811:148381. doi: 10.1016/j.brainres.2023.148381. Epub 2023 Apr 29. Brain Res. 2023. PMID: 37127174 Free PMC article.
-
Statistical parametric maps of ¹⁸F-FDG PET and 3-D autoradiography in the rat brain: a cross-validation study.Eur J Nucl Med Mol Imaging. 2011 Dec;38(12):2228-37. doi: 10.1007/s00259-011-1905-y. Epub 2011 Aug 27. Eur J Nucl Med Mol Imaging. 2011. PMID: 21874322
-
Imaging SERT Availability in a Rat Model of L-DOPA-Induced Dyskinesia.Mol Imaging Biol. 2020 Jun;22(3):634-642. doi: 10.1007/s11307-019-01418-2. Mol Imaging Biol. 2020. PMID: 31392531
-
The VMAT-2 inhibitor tetrabenazine affects effort-related decision making in a progressive ratio/chow feeding choice task: reversal with antidepressant drugs.PLoS One. 2014 Jun 17;9(6):e99320. doi: 10.1371/journal.pone.0099320. eCollection 2014. PLoS One. 2014. PMID: 24937131 Free PMC article.
References
-
- Davidson JR. First-line pharmacotherapy approaches for generalized anxiety disorder. J Clin Psychiatry. 2009;70(Suppl 2):25–31. - PubMed
-
- Nemeroff CB. The burden of severe depression: A review of diagnostic challenges and treatment alternatives. J Psychiatr Res. 2006 - PubMed
-
- Thase ME. Are SNRIs More Effective than SSRIs? A Review of the Current State of the Controversy. Psychopharmacol Bull. 2008;41:58–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

