Neuronal nitric oxide synthase and human vascular regulation
- PMID: 20447567
- PMCID: PMC2984617
- DOI: 10.1016/j.tcm.2010.02.007
Neuronal nitric oxide synthase and human vascular regulation
Abstract
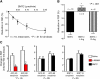
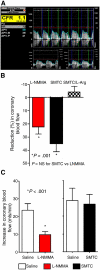
Vascular blood flow and its distribution among different vascular beds are regulated by changes in microvascular tone. Nitric oxide (NO) plays a key role in the local paracrine regulation of vessel tone both under resting conditions and when blood flow increases in response to agonist stimulation or increased shear stress. The conventional notion that endothelial NO synthase (eNOS)-derived NO is largely responsible for both effects has been challenged by first-in-human studies with a selective inhibitor of neuronal NOS (nNOS), S-methyl-l-thiocitrulline (SMTC). These studies reveal that SMTC causes a reduction in basal blood flow in the normal human forearm and coronary circulations (that is reversed by l-arginine), without affecting the eNOS-mediated vasodilatation elicited by acetylcholine, substance P, or increased shear stress. S-methyl-l-thiocitrulline also inhibits mental stress-induced vasodilatation. These results are consistent with a significant body of experimental studies suggesting that nNOS plays an important role in the local regulation of vessel tone in other species, independent of the effects of nNOS-derived NO in the central nervous system. These emerging data suggest that eNOS and nNOS have distinct roles in the physiologic local regulation of human microvascular tone in vivo and pave the way for further detailed investigation of the relative contribution of nNOS and eNOS in vascular regulation in human disease.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
References
-
- Bachetti T., Comini L., Curello S. Co-expression and modulation of neuronal and endothelial nitric oxide synthase in human endothelial cells. J Mol Cell Cardiol. 2004;37:939–945. - PubMed
-
- Bachmann S., Bosse H.M., Mundel P. Topography of nitric oxide synthesis by localizing constitutive NO synthase in mammalian kidney. Am J Physiol (Renal Fluid Electrolyte Physiol) 1995;37:F885–F898. - PubMed
-
- Bauser-Heaton H.D., Bohlen H.G. Cerebral microvascular dilation during hypotension and decreased oxygen tension: a role for nNOS. Am J Physiol Heart Circ Physiol. 2007;293:H2193–H2201. - PubMed
-
- Boulanger C.M., Heymes C., Benessiano J. Neuronal nitric oxide synthase is expressed in rat vascular smooth muscle cells: activation by angiotensin II in hypertension. Circ Res. 1998;83:1271–1278. - PubMed
-
- Brenman J.E., Chao D.S., Xia H., Aldape K., Bredt D.S. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

