Pitfalls in the sample preparation and analysis of N-acylethanolamines
- PMID: 20447930
- PMCID: PMC2936757
- DOI: 10.1194/jlr.D004606
Pitfalls in the sample preparation and analysis of N-acylethanolamines
Abstract
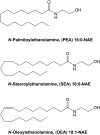
N-acylethanolamines (NAEs) are a group of lipid mediators synthesized in response to a number of physiological and pathological stimuli. Because of the low tissue concentrations of NAEs, analyses often include liquid extraction followed by solid-phase extraction and subsequent quantitation by LC/MS or GC/MS. Reported levels of NAEs vary considerably, however, and often no explanation is given for these discrepancies. Brought on by difficulties encountered during method development, the effects of using four different brands of silica-containing solid phase extraction (SPE) columns and five different brands of chloroform for sample preparation were investigated. Considerable variation in the retention and recoveries of seven NAEs and 2-arachidonoylglycerol existed between the SPE columns. Furthermore, it was found that some chloroforms contained quantifiable amounts of N-palmitoylethanolamine and N-stearoylethanolamine. Finally, it was found that use of one of the chloroforms resulted in a loss of N-oleoylethanolamine from solution due to addition of chlorine to the ω-9 bond. The identity of this reaction product was confirmed by LC-MS/MS and NMR. It is recommended that these aspects of sample preparation and analysis should be thoroughly validated during method development and the relevant information on specific brands used be reported in future communications in order to better estimate the validity of reported quantitative data.
Figures


Similar articles
-
Determination of endocannabinoids and N-acylethanolamines in human hair with LC-MS/MS and their relation to symptoms of depression, burnout, and anxiety.Talanta. 2020 Sep 1;217:121006. doi: 10.1016/j.talanta.2020.121006. Epub 2020 Apr 9. Talanta. 2020. PMID: 32498885
-
Quantitative profiling of endocannabinoids and related N-acylethanolamines in human CSF using nano LC-MS/MS.J Lipid Res. 2017 Mar;58(3):615-624. doi: 10.1194/jlr.D070433. Epub 2016 Dec 20. J Lipid Res. 2017. PMID: 27999147 Free PMC article.
-
Analysis of anandamide, an endogenous cannabinoid substance, and of other natural N-acylethanolamines.Prostaglandins Leukot Essent Fatty Acids. 1995 Oct;53(4):301-8. doi: 10.1016/0952-3278(95)90130-2. Prostaglandins Leukot Essent Fatty Acids. 1995. PMID: 8577784
-
A review of analytical methods for eicosanoids in brain tissue.J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Aug 1;964:50-64. doi: 10.1016/j.jchromb.2014.03.007. Epub 2014 Mar 14. J Chromatogr B Analyt Technol Biomed Life Sci. 2014. PMID: 24685838 Review.
-
Lipidomic Analysis of Endocannabinoid Signaling: Targeted Metabolite Identification and Quantification.Neural Plast. 2016;2016:2426398. doi: 10.1155/2016/2426398. Epub 2015 Dec 29. Neural Plast. 2016. PMID: 26839710 Free PMC article. Review.
Cited by
-
Analysis of ECs and related compounds in plasma: artifactual isomerization and ex vivo enzymatic generation of 2-MGs.J Lipid Res. 2014 May;55(5):966-77. doi: 10.1194/jlr.D043794. Epub 2014 Mar 7. J Lipid Res. 2014. PMID: 24610889 Free PMC article.
-
Estimation of reference intervals of five endocannabinoids and endocannabinoid related compounds in human plasma by two dimensional-LC/MS/MS.J Lipid Res. 2012 Mar;53(3):481-493. doi: 10.1194/jlr.M021378. Epub 2011 Dec 14. J Lipid Res. 2012. PMID: 22172516 Free PMC article.
-
Tissue storage affects lipidome profiling in comparison to in vivo microsampling approach.Sci Rep. 2018 May 3;8(1):6980. doi: 10.1038/s41598-018-25428-2. Sci Rep. 2018. PMID: 29725071 Free PMC article.
-
Determination of the Endocannabinoids Anandamide and 2-Arachidonoyl Glycerol with Gas Chromatography-Mass Spectrometry: Analytical and Preanalytical Challenges and Pitfalls.Med Cannabis Cannabinoids. 2018 Jun 12;1(1):9-18. doi: 10.1159/000489032. eCollection 2018 Jun. Med Cannabis Cannabinoids. 2018. PMID: 34676317 Free PMC article.
-
Dietary non-esterified oleic Acid decreases the jejunal levels of anorectic N-acylethanolamines.PLoS One. 2014 Jun 24;9(6):e100365. doi: 10.1371/journal.pone.0100365. eCollection 2014. PLoS One. 2014. PMID: 24959837 Free PMC article.
References
-
- Capasso R., Izzo A. A. 2008. Gastrointestinal regulation of food intake: general aspects and focus on anandamide and oleoylethanolamide. J. Neuroendocrinol. 20(Suppl. 1): 39–46. - PubMed
-
- Hansen H. S., Diep T. A. 2009. N-acylethanolamines, anandamide and food intake. Biochem. Pharmacol. 78: 553–560. - PubMed
-
- Cerrato S., Brazis P., Della Valle M. F., Miolo A., Puigdemont A. 2010. Effects of palmitoylethanolamide on immunologically induced histamine, PGD(2) and TNFalpha release from canine skin mast cells. Vet. Immunol. Immunopathol. 133: 9–15. - PubMed
-
- LoVerme J., Russo R., La R. G., Fu J., Farthing J., Mattace-Raso G., Meli R., Hohmann A., Calignano A., Piomelli D. 2006. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J. Pharmacol. Exp. Ther. 319: 1051–1061. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

