The visualisation of vitreous using surface modified poly(lactic-co-glycolic acid) microparticles
- PMID: 20447968
- PMCID: PMC2976469
- DOI: 10.1136/bjo.2009.163642
The visualisation of vitreous using surface modified poly(lactic-co-glycolic acid) microparticles
Abstract
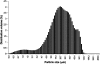
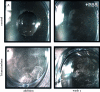
AIMS To demonstrate the potential use of in vitro poly(lactic-co-glycolic acid) (PLGA) microparticles in comparison with triamcinolone suspension to aid visualisation of vitreous during anterior and posterior vitrectomy. METHODS PLGA microparticles (diameter 10-60 microm) were fabricated using single and/or double emulsion technique(s) and used untreated or following the surface adsorption of a protein (transglutaminase). Particle size, shape, morphology and surface topography were assessed using scanning electron microscopy (SEM) and compared with a standard triamcinolone suspension. The efficacy of these microparticles to enhance visualisation of vitreous against the triamcinolone suspension was assessed using an in vitro set-up exploiting porcine vitreous. RESULTS Unmodified PLGA microparticles failed to adequately adhere to porcine vitreous and were readily washed out by irrigation. In contrast, modified transglutaminase-coated PLGA microparticles demonstrated a significant improvement in adhesiveness and were comparable to a triamcinolone suspension in their ability to enhance the visualisation of vitreous. This adhesive behaviour also demonstrated selectivity by not binding to the corneal endothelium. CONCLUSION The use of transglutaminase-modified biodegradable PLGA microparticles represents a novel method of visualising vitreous and aiding vitrectomy. This method may provide a distinct alternative for the visualisation of vitreous whilst eliminating the pharmacological effects of triamcinolone acetonide suspension.
Conflict of interest statement
Figures



Similar articles
-
Influence of the microencapsulation method and peptide loading on poly(lactic acid) and poly(lactic-co-glycolic acid) degradation during in vitro testing.J Control Release. 1998 Feb 12;51(2-3):327-41. doi: 10.1016/s0168-3659(97)00188-0. J Control Release. 1998. PMID: 9685930
-
rhBMP-2 release from injectable poly(DL-lactic-co-glycolic acid)/calcium-phosphate cement composites.J Bone Joint Surg Am. 2003;85-A Suppl 3:75-81. doi: 10.2106/00004623-200300003-00013. J Bone Joint Surg Am. 2003. PMID: 12925613
-
Comparative study of poly (lactic-co-glycolic acid)-poly ethyleneimine-plasmid DNA microparticles prepared using double emulsion methods.J Microencapsul. 2008 Feb;25(1):1-12. doi: 10.1080/02652040701659347. J Microencapsul. 2008. PMID: 18188727
-
Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model.Eur J Pharmacol. 2005 Mar 28;511(2-3):191-8. doi: 10.1016/j.ejphar.2005.02.019. Eur J Pharmacol. 2005. PMID: 15792788
-
Rational design, fabrication, characterization and in vitro testing of biodegradable microparticles that generate targeted and sustained transgene expression in HepG2 liver cells.J Drug Target. 2011 Jul;19(6):393-408. doi: 10.3109/1061186X.2010.504263. Epub 2010 Aug 3. J Drug Target. 2011. PMID: 20681752 Free PMC article.
Cited by
-
Optimising poly(lactic-co-glycolic acid) microparticle fabrication using a Taguchi orthogonal array design-of-experiment approach.PLoS One. 2019 Sep 26;14(9):e0222858. doi: 10.1371/journal.pone.0222858. eCollection 2019. PLoS One. 2019. PMID: 31557205 Free PMC article.
References
-
- Johnston RL, Taylor H, Smith R, et al. The cataract National Dataset Electronic Multi-centre Audit of 55 567 Operations: variation in posterior capsule rupture rates between surgeons. Eye (Lond) 2009. Aug 14. [Epub ahead of print]. doi: 10.1038/eye.2009.195 - DOI - PubMed
-
- Pingree MF, Crandall AS, Olson RJ. Cataract surgery complications in 1 year at an academic institution. J Cataract Refract Surg 1999;25:705–8 - PubMed
-
- Burk SE, Da Mata AP, Snyder ME, et al. Visualizing vitreous using Kenalog suspension. J Cataract Refract Surg 2003;29:645–51 - PubMed
-
- Yamakiri K, Uchino E, Kimura K, et al. Intracameral triamcinolone helps to visualize and remove the vitreous body in anterior chamber in cataract surgery. Am J Ophthalmol 2004;138:650–2 - PubMed
-
- Oh JY, Wee WR, Lee JH, et al. Short-term effect of intracameral triamcinolone acetonide on corneal endothelium using the rabbit model. Eye 2007;21:812–18 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
