IgG glycan hydrolysis attenuates ANCA-mediated glomerulonephritis
- PMID: 20448018
- PMCID: PMC3152232
- DOI: 10.1681/ASN.2009090984
IgG glycan hydrolysis attenuates ANCA-mediated glomerulonephritis
Abstract
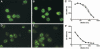
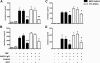
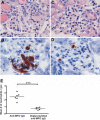
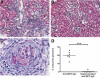
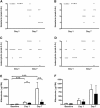
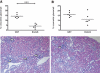
Anti-neutrophil cytoplasmic autoantibodies (ANCA) directed against myeloperoxidase (MPO) and proteinase 3 (Pr3) are considered pathogenic in ANCA-associated necrotizing and crescentic glomerulonephritis (NCGN) and vasculitis. Modulation of ANCA IgG glycosylation may potentially reduce its pathogenicity by abolishing Fc receptor-mediated activation of leukocytes and complement. Here, we investigated whether IgG hydrolysis by the bacterial enzyme endoglycosidase S (EndoS) attenuates ANCA-mediated NCGN. In vitro, treatment of ANCA IgG with EndoS significantly attenuated ANCA-mediated neutrophil activation without affecting antigen-binding capacity. In a mouse model of anti-MPO IgG/LPS-induced NCGN, we induced disease with either unmodified or EndoS-treated (deglycosylated) anti-MPO IgG. In separate experiments, we administered EndoS systemically after disease induction with unmodified anti-MPO IgG. Pretreatment of anti-MPO IgG with EndoS reduced hematuria, leukocyturia, and albuminuria and attenuated both neutrophil influx and formation of glomerular crescents. After inducing disease with unmodified anti-MPO IgG, systemic treatment with EndoS reduced albuminuria and glomerular crescent formation when initiated after 3 but not 24 hours. In conclusion, IgG glycan hydrolysis by EndoS attenuates ANCA-induced neutrophil activation in vitro and prevents induction of anti-MPO IgG/LPS-mediated NCGN in vivo. Systemic treatment with EndoS early after disease induction attenuates the development of disease. Thus, modulation of IgG glycosylation is a promising strategy to interfere with ANCA-mediated inflammatory processes.
Figures

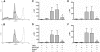


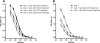
References
-
- Kallenberg CG, Heeringa P, Stegeman CA: Mechanisms of disease: Pathogenesis and treatment of ANCA-associated vasculitides. Nat Clin Pract Rheumatol 2: 661–670, 2006 - PubMed
-
- Franssen CF, Stegeman CA, Kallenberg CG, Gans RO, De Jong PE, Hoorntje SJ, Tervaert JW: Antiproteinase 3- and antimyeloperoxidase-associated vasculitis. Kidney Int 57: 2195–2206, 2000 - PubMed
-
- Turnbull J, Harper L: Adverse effects of therapy for ANCA-associated vasculitis. Best Pract Res Clin Rheumatol 23: 391–401, 2009 - PubMed
-
- van Timmeren MM, Chen M, Heeringa P: Review article: Pathogenic role of complement activation in anti-neutrophil cytoplasmic auto-antibody-associated vasculitis. Nephrology (Carlton) 14: 16–25, 2009 - PubMed
-
- Heeringa P, Huugen D, Tervaert JW: Anti-neutrophil cytoplasmic autoantibodies and leukocyte-endothelial interactions: A sticky connection? Trends Immunol 26: 561–564, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

