Epigenetic alterations in aging
- PMID: 20448029
- PMCID: PMC2928596
- DOI: 10.1152/japplphysiol.00238.2010
Epigenetic alterations in aging
Abstract
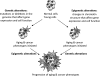
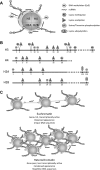
Aging is a multifaceted process characterized by genetic and epigenetic changes in the genome. The genetic component of aging received initially all of the attention. Telomere attrition and accumulation of mutations due to a progressive deficiency in the repair of DNA damage with age remain leading causes of genomic instability. However, epigenetic mechanisms have now emerged as key contributors to the alterations of genome structure and function that accompany aging. The three pillars of epigenetic regulation are DNA methylation, histone modifications, and noncoding RNA species. Alterations of these epigenetic mechanisms affect the vast majority of nuclear processes, including gene transcription and silencing, DNA replication and repair, cell cycle progression, and telomere and centromere structure and function. Here, we summarize the lines of evidence indicating that these epigenetic defects might represent a major factor in the pathophysiology of aging and aging-related diseases, especially cancer.
Figures



References
-
- Ahuja N, Li Q, Mohan AL, Baylin SB, Issa JP. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res 58: 5489–5494, 1998 - PubMed
-
- Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 316: 744–747, 2007 - PubMed
-
- Ballestar E, Esteller M. Methyl-CpG-binding proteins in cancer: blaming the DNA methylation messenger. Biochem Cell Biol 83: 374–384, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

