Redox biochemistry of hydrogen sulfide
- PMID: 20448039
- PMCID: PMC2903356
- DOI: 10.1074/jbc.R110.128363
Redox biochemistry of hydrogen sulfide
Abstract
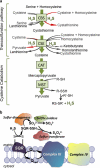
H(2)S, the most recently discovered gasotransmitter, might in fact be the evolutionary matriarch of this family, being both ancient and highly reduced. Disruption of gamma-cystathionase in mice leads to cardiovascular dysfunction and marked hypertension, suggesting a key role for this enzyme in H(2)S production in the vasculature. However, patients with inherited deficiency in gamma-cystathionase apparently do not present vascular pathology. A mitochondrial pathway disposes sulfide and couples it to oxidative phosphorylation while also exposing cytochrome c oxidase to this metabolic poison. This report focuses on the biochemistry of H(2)S biogenesis and clearance, on the molecular mechanisms of its action, and on its varied biological effects.
Figures
References
-
- Vorobets V. S., Kovach S. K., Kolbasov G. Y. (2002) Russian J. Appl. Chem. 75, 229–234
-
- Li C., Love G. D., Lyons T. W., Fike D. A., Sessions A. L., Chu X. (2010) Science 328, 80–83 - PubMed
-
- Grice K., Cao C., Love G. D., Böttcher M. E., Twitchett R. J., Grosjean E., Summons R. E., Turgeon S. C., Dunning W., Jin Y. (2005) Science 307, 706–709 - PubMed
-
- Furne J., Saeed A., Levitt M. D. (2008) Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1479–R1485 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

