Placenta growth factor-induced early growth response 1 (Egr-1) regulates hypoxia-inducible factor-1alpha (HIF-1alpha) in endothelial cells
- PMID: 20448047
- PMCID: PMC2898343
- DOI: 10.1074/jbc.M110.119495
Placenta growth factor-induced early growth response 1 (Egr-1) regulates hypoxia-inducible factor-1alpha (HIF-1alpha) in endothelial cells
Abstract
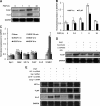
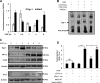
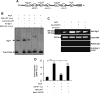
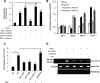
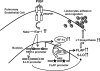
Leukotrienes, the lipid inflammatory products derived from arachidonic acid, are involved in the pathogenesis of respiratory and cardiovascular diseases and reactive airway disease in sickle cell disease. Placenta growth factor (PlGF), elaborated from erythroid cells, increased the mRNA expression of 5-lipoxygenase and 5-lipoxygenase-activating protein (FLAP) in human pulmonary microvascular endothelial cells. PlGF-induced both promoter activity and mRNA expression of hypoxia-inducible factor-1alpha (HIF-1alpha), which was abrogated by early growth response-1 (EGR-1) small interfering RNA. PlGF showed a temporal reciprocal relationship in the mRNA levels of EGR-1 and NAB2, the latter a repressor of Egr-1. Moreover, Nab2, but not mutant Nab2, significantly reduced promoter activity and mRNA expression of HIF-1alpha and also reduced expression of the HIF-1alpha target gene FLAP. Furthermore, overexpression of Egr-1 led to increased promoter activities for both HIF-1alpha and FLAP in the absence of PlGF. Additionally, the Egr-1-mediated induction of HIF-1alpha and FLAP promoters was reduced to basal levels by EGR-1 small interfering RNA. The binding of Egr-1 to HIF-1alpha promoter was corroborated by electrophoretic mobility shift assay and chromatin immunoprecipitation assay, which showed increased Egr-1 binding to the HIF-1alpha promoter in response to PlGF stimulation. These studies provide a novel mechanism for PlGF-mediated regulation of HIF-1alpha via Egr-1, which results in increased FLAP expression. This study provides a new therapeutic target, namely Egr-1, for attenuation of elevated leukotriene levels in patients with sickle cell disease and other inflammatory diseases.
Figures
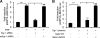
Similar articles
-
Placenta growth factor induces 5-lipoxygenase-activating protein to increase leukotriene formation in sickle cell disease.Blood. 2009 Jan 29;113(5):1129-38. doi: 10.1182/blood-2008-07-169821. Epub 2008 Oct 22. Blood. 2009. PMID: 18945963 Free PMC article.
-
Erythropoietin-mediated expression of placenta growth factor is regulated via activation of hypoxia-inducible factor-1α and post-transcriptionally by miR-214 in sickle cell disease.Biochem J. 2015 Jun 15;468(3):409-23. doi: 10.1042/BJ20141138. Epub 2015 Apr 16. Biochem J. 2015. PMID: 25876995 Free PMC article.
-
Hypoxia-mediated expression of 5-lipoxygenase-activating protein involves HIF-1alpha and NF-kappaB and microRNAs 135a and 199a-5p.J Immunol. 2010 Apr 1;184(7):3878-88. doi: 10.4049/jimmunol.0902594. Epub 2010 Mar 1. J Immunol. 2010. PMID: 20194722
-
Early growth response-1, a dynamic conduit in cardiovascular disease.Front Cardiovasc Med. 2024 Nov 15;11:1487668. doi: 10.3389/fcvm.2024.1487668. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39619154 Free PMC article. Review.
-
Early growth response-1: Key mediators of cell death and novel targets for cardiovascular disease therapy.Front Cardiovasc Med. 2023 Mar 28;10:1162662. doi: 10.3389/fcvm.2023.1162662. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37057102 Free PMC article. Review.
Cited by
-
Tracing the cis-regulatory changes underlying the endometrial control of placental invasion.Proc Natl Acad Sci U S A. 2022 Feb 8;119(6):e2111256119. doi: 10.1073/pnas.2111256119. Proc Natl Acad Sci U S A. 2022. PMID: 35110402 Free PMC article.
-
Cigarette smoke extract induces placental growth factor release from human bronchial epithelial cells via ROS/MAPK (ERK-1/2)/Egr-1 axis.Int J Chron Obstruct Pulmon Dis. 2016 Dec 2;11:3031-3042. doi: 10.2147/COPD.S120849. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27980400 Free PMC article.
-
Epigenetic control of hypoxia inducible factor-1α-dependent expression of placental growth factor in hypoxic conditions.Epigenetics. 2014 Apr;9(4):600-10. doi: 10.4161/epi.27835. Epub 2014 Feb 6. Epigenetics. 2014. PMID: 24504136 Free PMC article.
-
SINHCAF/FAM60A and SIN3A specifically repress HIF-2α expression.Biochem J. 2018 Jun 29;475(12):2073-2090. doi: 10.1042/BCJ20170945. Biochem J. 2018. PMID: 29784889 Free PMC article.
-
Reverse translation of phase I biomarker findings links the activity of angiotensin-(1-7) to repression of hypoxia inducible factor-1α in vascular sarcomas.BMC Cancer. 2012 Sep 11;12:404. doi: 10.1186/1471-2407-12-404. BMC Cancer. 2012. PMID: 22963500 Free PMC article.
References
-
- Lewis R. A., Austen K. F., Soberman R. J. (1990) N. Engl. J. Med. 323, 645–655 - PubMed
-
- Samuelsson B., Dahlén S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. (1987) Science 237, 1171–1176 - PubMed
-
- Zhao L., Moos M. P., Gräbner R., Pédrono F., Fan J., Kaiser B., John N., Schmidt S., Spanbroek R., Lötzer K., Huang L., Cui J., Rader D. J., Evans J. F., Habenicht A. J., Funk C. D. (2004) Nat. Med. 10, 966–973 - PubMed
-
- Funk C. D. (2001) Science 294, 1871–1875 - PubMed
-
- Peters-Golden M., Henderson W. R., Jr. (2007) N. Engl. J. Med. 357, 1841–1854 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

