Neutral sphingomyelinase 2: a novel target in cigarette smoke-induced apoptosis and lung injury
- PMID: 20448054
- PMCID: PMC3095936
- DOI: 10.1165/rcmb.2009-0422OC
Neutral sphingomyelinase 2: a novel target in cigarette smoke-induced apoptosis and lung injury
Abstract
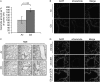
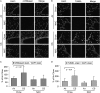
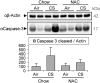
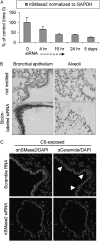
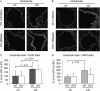
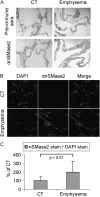
Chronic obstructive pulmonary disease (COPD) is caused by exposure to cigarette smoke (CS). One mechanism of CS-induced lung injury is aberrant generation of ceramide, which leads to elevated apoptosis of epithelial and endothelial cells in the alveolar spaces. Recently, we discovered that CS-induced ceramide generation and apoptosis in pulmonary cells is governed by neutral sphingomyelinase (nSMase) 2. In the current experiments, we expanded our studies to investigate whether nSMase2 governs ceramide generation and apoptosis in vivo using rodent and human models of CS-induced lung injury. We found that exposure of mice or rats to CS leads to colocalizing elevations of ceramide levels and terminal deoxynucleotidyl transferase mediated X-dUTP nick end labeling-positive cells in lung tissues. These increases are nSMase2 dependent, and are abrogated by treatment with N-acetyl cysteine or anti-nSMase2 small interfering RNA (siRNA). We further showed that mice that are heterozygous for nSMase2 demonstrate significant decrease in ceramide generation after CS exposure, whereas acidic sphingomyelinase (aSMase) knockout mice maintain wild-type ceramide levels, confirming our previous findings (in human airway epithelial cells) that only nSMase2, and not aSMase, is activated by CS exposure. Lastly, we found that lung tissues from patients with emphysema (smokers) display significantly higher levels of nSMase2 expression compared with lung tissues from healthy control subjects. Taken together, these data establish the central in vivo role of nSMase2 in ceramide generation, aberrant apoptosis, and lung injury under CS exposure, underscoring its promise as a novel target for the prevention of CS-induced airspace destruction.
Figures

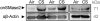

Similar articles
-
Neutral sphingomyelinase 2 is activated by cigarette smoke to augment ceramide-induced apoptosis in lung cell death.Am J Physiol Lung Cell Mol Physiol. 2009 Jul;297(1):L125-33. doi: 10.1152/ajplung.00031.2009. Epub 2009 Apr 24. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19395669 Free PMC article.
-
Src regulates cigarette smoke-induced ceramide generation via neutral sphingomyelinase 2 in the airway epithelium.Am J Respir Cell Mol Biol. 2015 Jun;52(6):738-48. doi: 10.1165/rcmb.2014-0122OC. Am J Respir Cell Mol Biol. 2015. PMID: 25347576 Free PMC article.
-
Ceramide-1-phosphate inhibits cigarette smoke-induced airway inflammation.Eur Respir J. 2015 Jun;45(6):1669-80. doi: 10.1183/09031936.00080014. Epub 2015 Jan 22. Eur Respir J. 2015. PMID: 25614161
-
Lung cancer and lung injury: the dual role of ceramide.Handb Exp Pharmacol. 2013;(216):93-113. doi: 10.1007/978-3-7091-1511-4_5. Handb Exp Pharmacol. 2013. PMID: 23563653 Free PMC article. Review.
-
Lung injury and lung cancer caused by cigarette smoke-induced oxidative stress: Molecular mechanisms and therapeutic opportunities involving the ceramide-generating machinery and epidermal growth factor receptor.Antioxid Redox Signal. 2014 Nov 20;21(15):2149-74. doi: 10.1089/ars.2013.5469. Epub 2014 Jul 1. Antioxid Redox Signal. 2014. PMID: 24684526 Free PMC article. Review.
Cited by
-
New Therapeutic Options in Pulmonal Diseases: Sphingolipids and Modulation of Sphingolipid Metabolism.Handb Exp Pharmacol. 2024;284:289-312. doi: 10.1007/164_2023_700. Handb Exp Pharmacol. 2024. PMID: 37922034 Review.
-
Neutral sphingomyelinase 2 activity and protein stability are modulated by phosphorylation of five conserved serines.J Biol Chem. 2012 Jan 2;287(1):514-522. doi: 10.1074/jbc.M111.315481. Epub 2011 Nov 10. J Biol Chem. 2012. PMID: 22074919 Free PMC article.
-
Vam3, a resveratrol dimer, inhibits cigarette smoke-induced cell apoptosis in lungs by improving mitochondrial function.Acta Pharmacol Sin. 2014 Jun;35(6):779-91. doi: 10.1038/aps.2014.17. Epub 2014 Apr 21. Acta Pharmacol Sin. 2014. PMID: 24747163 Free PMC article.
-
Sphingolipids in the DNA damage response.Adv Biol Regul. 2015 May;58:38-52. doi: 10.1016/j.jbior.2014.11.001. Epub 2014 Nov 18. Adv Biol Regul. 2015. PMID: 25434743 Free PMC article. Review.
-
Extracellular vesicular delivery of ceramides from pulmonary macrophages to endothelial cells facilitates chronic obstructive pulmonary disease.Cell Commun Signal. 2025 Mar 7;23(1):124. doi: 10.1186/s12964-025-02125-y. Cell Commun Signal. 2025. PMID: 40055817 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

