Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib
- PMID: 20448206
- PMCID: PMC2917780
- DOI: 10.1161/ATVBAHA.110.207142
Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib
Abstract
Objective: To examine the effects of treatments with niacin or anacetrapib (an inhibitor of cholesteryl ester transfer protein) on the ability of high-density lipoprotein (HDL) to promote net cholesterol efflux and reduce toll-like receptor-mediated inflammation in macrophages.
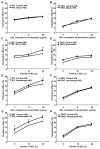
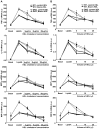
Methods and results: A total of 18 patients received niacin, 2 g/d, for 4 weeks; 20 patients received anacetrapib, 300 mg/d, for 8 weeks; and 2 groups (n=4 and n=5 patients) received placebo. HDL samples were isolated by polyethylene glycol precipitation or ultracentrifugation, tested for the ability to promote cholesterol efflux in cholesterol-loaded THP-I or mouse peritoneal macrophages, or used to pretreat macrophages, followed by lipopolysaccharide exposure. HDL cholesterol levels were increased by 30% in response to niacin and by approximately 100% in response to anacetrapib. Niacin treatment increased HDL-mediated net cholesterol efflux from foam cells, primarily by increasing HDL concentration, whereas anacetrapib treatment increased cholesterol efflux by both increasing HDL concentration and causing increased efflux at matched HDL concentrations. The increased efflux potential of anacetrapib-HDL was more prominent at higher HDL cholesterol concentrations (>12 microg/mL), which was associated with an increased content of lecithin-cholesterol acyltransferase (LCAT) and apolipoprotein E and completely dependent on the expression of ATP binding cassette transporters (ABCA1 and ABCG1). Potent antiinflammatory effects of HDL were observed at low HDL concentrations (3 to 20 microg/mL) and were partly dependent on the expression of ABCA1 and ABCG1. All HDL preparations showed similar antiinflammatory effects, proportionate to the HDL cholesterol concentration.
Conclusions: Niacin treatment caused a moderate increase in the ability of HDL to promote net cholesterol efflux, whereas inhibition of cholesteryl ester transfer protein via anacetrapib led to a more dramatic increase in association with enhanced particle functionality at higher HDL concentrations. All HDLs exhibited potent ability to suppress macrophage toll-like receptor 4-mediated inflammatory responses, in a process partly dependent on cholesterol efflux via ABCA1 and ABCG1.
Conflict of interest statement
Figures

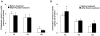
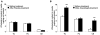
Similar articles
-
Differential effects of fenofibrate and extended-release niacin on high-density lipoprotein particle size distribution and cholesterol efflux capacity in dyslipidemic patients.J Clin Lipidol. 2013 Sep-Oct;7(5):414-22. doi: 10.1016/j.jacl.2013.06.007. Epub 2013 Jun 27. J Clin Lipidol. 2013. PMID: 24079282 Clinical Trial.
-
HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoE- and ABCG1-dependent pathway.J Clin Invest. 2006 May;116(5):1435-42. doi: 10.1172/JCI27602. J Clin Invest. 2006. PMID: 16670775 Free PMC article.
-
HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export.Circ Res. 2015 Mar 27;116(7):1133-42. doi: 10.1161/CIRCRESAHA.116.305485. Epub 2015 Jan 14. Circ Res. 2015. PMID: 25589556
-
Patient considerations and clinical impact of cholesteryl ester transfer protein inhibitors in the management of dyslipidemia: focus on anacetrapib.Vasc Health Risk Manag. 2012;8:483-93. doi: 10.2147/VHRM.S29010. Epub 2012 Aug 23. Vasc Health Risk Manag. 2012. PMID: 22977305 Free PMC article. Review.
-
Cholesteryl ester transfer-protein modulator and inhibitors and their potential for the treatment of cardiovascular diseases.Vasc Health Risk Manag. 2012;8:323-31. doi: 10.2147/VHRM.S25238. Epub 2012 May 15. Vasc Health Risk Manag. 2012. PMID: 22661899 Free PMC article. Review.
Cited by
-
Hematopoietic stem/progenitor cell proliferation and differentiation is differentially regulated by high-density and low-density lipoproteins in mice.PLoS One. 2012;7(11):e47286. doi: 10.1371/journal.pone.0047286. Epub 2012 Nov 7. PLoS One. 2012. PMID: 23144813 Free PMC article.
-
Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis.J Clin Invest. 2011 Jul;121(7):2921-31. doi: 10.1172/JCI57275. Epub 2011 Jun 6. J Clin Invest. 2011. PMID: 21646721 Free PMC article.
-
HDL-targeted therapies: progress, failures and future.Nat Rev Drug Discov. 2014 Jun;13(6):445-64. doi: 10.1038/nrd4279. Epub 2014 May 23. Nat Rev Drug Discov. 2014. PMID: 24854407 Review.
-
Cholesterol-Ester Transfer Protein Alters M1 and M2 Macrophage Polarization and Worsens Experimental Elastase-Induced Pulmonary Emphysema.Front Immunol. 2021 Jul 21;12:684076. doi: 10.3389/fimmu.2021.684076. eCollection 2021. Front Immunol. 2021. PMID: 34367144 Free PMC article.
-
The not-so-simple HDL story: Is it time to revise the HDL cholesterol hypothesis?Nat Med. 2012 Sep;18(9):1344-6. doi: 10.1038/nm.2937. Nat Med. 2012. PMID: 22961164 No abstract available.
References
-
- Rader DJ. Mechanisms of disease: HDL metabolism as a target for novel therapies. Nat Clin Pract Cardiovasc Med. 2007;4:102–109. - PubMed
-
- Joy T, Hegele RA. Is raising HDL a futile strategy for atheroprotection? Nat Rev Drug Discov. 2008;7:143–155. - PubMed
-
- Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. - PubMed
-
- Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald W. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–1255. - PubMed
-
- Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

