Early mitochondrial dysfunction in electron transfer activity and reactive oxygen species generation after cardiac arrest
- PMID: 20449909
- PMCID: PMC3315374
- DOI: 10.1097/ccm.0b013e31818a8a51
Early mitochondrial dysfunction in electron transfer activity and reactive oxygen species generation after cardiac arrest
Abstract
Objective: Mitochondrial biology appears central to many conditions that progress to death but remains poorly characterized after cardiac arrest. Mitochondrial dysfunction in electron transfer and reactive oxygen species leakage during ischemia may lead to downstream events including mitochondrial protein oxidation, tyrosine nitrosylation, cytochrome c loss, and eventual death. We sought to better define early fixed alterations in these mitochondrial functions after whole animal cardiac arrest.
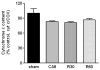
Methods: We used a murine model of 8 mins of untreated KCl-induced cardiac arrest followed by resuscitation and return of spontaneous circulation to study mitochondrial functions in four groups of animals: 1) after 8 min cardiac arrest (CA8) but no resuscitation, 2) 30 min postreturn of spontaneous circulation (R30), 3) 60 min postreturn of spontaneous circulation (R60), and in 4) shams. Heart mitochondria were immediately harvested, isolated, and stored at -80 degrees C for later spectrophotometric measurements of electron transfer activities and reactive oxygen species leakage using appropriate substrates and inhibitors. Mitochondrial cytochrome c content and tyrosine nitration were analyzed by Western blot and densitometry.
Results: A significant reactive oxygen species leakage from complex I was evident after just 8 min of cardiac arrest (CA8 group, p < .05), which was followed by a progressive reduction in complex I electron transfer activity (CA8 > R30 > R60). In contrast, complex II and II-III activities appeared more resistant to ischemia at the time points evaluated. Early changes in a approximately 50 kDa and approximately 25 kDa protein were observed in tyrosine nitration along with a loss of cytochrome c.
Conclusions: A relatively "orderly" process of mitochondrial dysfunction progresses during ischemia and reperfusion. Changes in mitochondrial reactive oxygen species generation and electron transfer from complex I occur along with tyrosine nitrosylation and loss of cytochrome c; these may represent important new targets for future human therapies.
Figures



References
-
- Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–470. - PubMed
-
- Mayevsky A, Chance B. Oxidation-reduction states of NADH in vivo: from animals to clinical use. Mitochondrion. 2007;7:330–339. - PubMed
-
- Borutaite V, Mildaziene V, Brown GC, et al. Control and kinetic analysis of ischemia-damaged heart mitochondria: which parts of the oxidative phosphorylation system are affected by ischemia? Biochim Biophys Acta. 1995;1272:154–158. - PubMed
-
- Vanden Hoek TL, Li C, Shao Z, et al. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol. 1997;29:2571–2583. - PubMed
-
- Qin Y, Vanden Hoek TL, Wojcik K, et al. Caspase-dependent cytochrome c release and cell death in chick cardiomyocytes after simulated ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2004;286:H2280–2286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

