Impact of mutation on proton transfer reactions in ketosteroid isomerase: insights from molecular dynamics simulations
- PMID: 20450180
- PMCID: PMC2896286
- DOI: 10.1021/ja102714u
Impact of mutation on proton transfer reactions in ketosteroid isomerase: insights from molecular dynamics simulations
Abstract
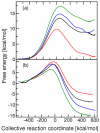
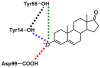
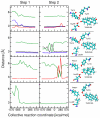
The two proton transfer reactions catalyzed by ketosteroid isomerase (KSI) involve a dienolate intermediate stabilized by hydrogen bonds with Tyr14 and Asp99. Molecular dynamics simulations based on an empirical valence bond model are used to examine the impact of mutating these residues on the hydrogen-bonding patterns, conformational changes, and van der Waals and electrostatic interactions during the proton transfer reactions. While the rate constants for the two proton transfer steps are similar for wild-type (WT) KSI, the simulations suggest that the rate constant for the first proton transfer step is smaller in the mutants due to the significantly higher free energy of the dienolate intermediate relative to the reactant. The calculated rate constants for the mutants D99L, Y14F, and Y14F/D99L relative to WT KSI are qualitatively consistent with the kinetic experiments indicating a significant reduction in the catalytic rates along the series of mutants. In the simulations, WT KSI retained two hydrogen-bonding interactions between the substrate and the active site, while the mutants typically retained only one hydrogen-bonding interaction. A new hydrogen-bonding interaction between the substrate and Tyr55 was observed in the double mutant, leading to the prediction that mutation of Tyr55 will have a greater impact on the proton transfer rate constants for the double mutant than for WT KSI. The electrostatic stabilization of the dienolate intermediate relative to the reactant was greater for WT KSI than for the mutants, providing a qualitative explanation for the significantly reduced rates of the mutants. The active site exhibited restricted motion during the proton transfer reactions, but small conformational changes occurred to facilitate the proton transfer reactions by strengthening the hydrogen-bonding interactions and by bringing the proton donor and acceptor closer to each other with the proper orientation for proton transfer. Thus, these calculations suggest that KSI forms a preorganized active site but that the structure of this preorganized active site is altered upon mutation. Moreover, small conformational changes due to stochastic thermal motions are required within this preorganized active site to facilitate the proton transfer reactions.
Figures
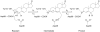
References
-
- Wu ZR, Ebrahimian S, Zawrotny ME, Thornburg LD, Perez-Alvarado GC, Brothers P, Pollack RM, Summers MF. Science. 1997;276:415–418. - PubMed
-
- Choi G, Ha N-C, Kim SW, Kim D-H, Park S, Oh B-H, Choi KY. Biochemistry. 2000;39:903–909. - PubMed
-
- Mildvan AS, Massiah MA, Harris TK, Marks GT, Harrison DHT, Viragh C, Reddy PM, Kovach IM. J. Mol. Struct. 2002;615:163–175.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

