Proteoglycomics: recent progress and future challenges
- PMID: 20450439
- PMCID: PMC3133783
- DOI: 10.1089/omi.2009.0123
Proteoglycomics: recent progress and future challenges
Abstract
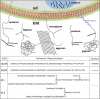
Proteoglycomics is a systematic study of structure, expression, and function of proteoglycans, a posttranslationally modified subset of a proteome. Although relying on the established technologies of proteomics and glycomics, proteoglycomics research requires unique approaches for elucidating structure-function relationships of both proteoglycan components, glycosaminoglycan chain, and core protein. This review discusses our current understanding of structure and function of proteoglycans, major players in the development, normal physiology, and disease. A brief outline of the proteoglycomic sample preparation and analysis is provided along with examples of several recent proteoglycomic studies. Unique challenges in the characterization of glycosaminoglycan component of proteoglycans are discussed, with emphasis on the many analytical tools used and the types of information they provide.
Figures


References
-
- Akama T.O. Nishida K. Nakayama J. Watanabe H. Ozaki K. Nakamura T., et al. Macular corneal dystrophy type I and type II are caused by distinct mutations in a new sulphotransferase gene. Nat Genet. 2000;26:237–241. - PubMed
-
- Amon S. Zamfir A.D. Rizzi A. Glycosylation analysis of glycoproteins and proteoglycans using capillary electrophoresis-mass spectrometry strategies. Electrophoresis. 2008;29:2485–2507. - PubMed
-
- Aono S. Tokita Y. Shuo T. Yamauchi S. Matsui F. Nakanishi K., et al. Glycosylation site for chondroitin sulfate on the neural part-time proteoglycan, neuroglycan C. J Biol Chem. 2004;279:46536–46541. - PubMed
-
- Armengaud J. A perfect genome annotation is within reach with the proteomics and genomics alliance. Curr Opin Microbiol. 2009;12:292–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

