Mitochondrial dysfunction may explain the cardiomyopathy of chronic iron overload
- PMID: 20450972
- PMCID: PMC2900522
- DOI: 10.1016/j.freeradbiomed.2010.04.033
Mitochondrial dysfunction may explain the cardiomyopathy of chronic iron overload
Abstract
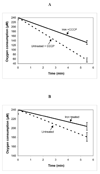
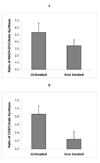
In patients with hemochromatosis, cardiac dysfunction may appear years after they have reached a state of iron overload. We hypothesized that cumulative iron-catalyzed oxidant damage to mitochondrial DNA (mtDNA) might explain the cardiomyopathy of chronic iron overload. Mice were given repetitive injections of iron dextran for a total of 4weeks after which the iron-loaded mice had elevated cardiac iron, modest cardiac hypertrophy, and cardiac dysfunction. qPCR amplification of near-full-length ( approximately 16kb) mtDNA revealed >50% loss of full-length product, whereas amounts of a qPCR product of a nuclear gene (13kb region of beta globin) were unaffected. Quantitative rtPCR analyses revealed 60-70% loss of mRNA for proteins encoded by mtDNA with no change in mRNA abundance for nuclear-encoded respiratory subunits. These changes coincided with proportionate reductions in complex I and IV activities and decreased respiration of isolated cardiac mitochondria. We conclude that chronic iron overload leads to cumulative iron-mediated damage to mtDNA and impaired synthesis of mitochondrial respiratory chain subunits. The resulting respiratory dysfunction may explain the slow development of cardiomyopathy in chronic iron overload and similar accumulation of damage to mtDNA may also explain the mitochondrial dysfunction observed in slowly progressing diseases such as neurodegenerative disorders.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Quinlan GJ, Evans TW, Gutteridge JM. Iron and redox status of the lungs. Free Radic. Biol. Med. 2002;33:1306–1313. - PubMed
-
- Ernst SH, Stuart L. Formation, prevention, and repair of DNA damage by iron/hydrogen peroxide. J. Biol. Chem. 1997;272:19095–19098. - PubMed
-
- Graf E, Mahoney JR, Bryant RG, Eaton JW. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J. Biol. Chem. 1984;259:3620–3624. - PubMed
-
- Loschen G, Azzi A. Proceedings: Formation of oxygen radicals and hydrogen peroxide in mitochondrial membranes. Hoppe Seylers Z Physiol Chem. 1974;355:1226. - PubMed
-
- Bartfay WJ, Bartfay E. Iron-overload cardiomyopathy: evidence for a free radical--mediated mechanism of injury and dysfunction in a murine model. Biol. Res. Nurs. 2000;2:49–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

