Skeletal muscle excitation-contraction coupling is independent of a conserved heptad repeat motif in the C-terminus of the DHPRbeta(1a) subunit
- PMID: 20451250
- PMCID: PMC2896708
- DOI: 10.1016/j.ceca.2010.04.003
Skeletal muscle excitation-contraction coupling is independent of a conserved heptad repeat motif in the C-terminus of the DHPRbeta(1a) subunit
Abstract
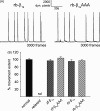
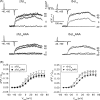
In skeletal muscle excitation-contraction (EC) coupling the sarcolemmal L-type Ca(2+) channel or 1,4-dihydropyridine receptor (DHPR) transduces the membrane depolarization signal to the sarcoplasmic Ca(2+) release channel RyR1 via protein-protein interaction. While it is evident that the pore-forming and voltage-sensing DHPRalpha(1S) subunit is essential for this process, the intracellular DHPRbeta(1a) subunit was also shown to be indispensable. We previously found that the beta(1a) subunit is essential to target the DHPR into groups of four (tetrads) opposite the RyR1 homotetramers, a prerequisite for skeletal muscle EC coupling. Earlier, a unique hydrophobic heptad repeat motif (Lcdots, three dots, centeredVcdots, three dots, centeredV) in the C-terminus of beta(1a) was postulated by others to be essential for skeletal muscle EC coupling, as substitution of these residues with alanines resulted in 80% reduction of RyR1 Ca(2+) release. Therefore, we wanted to address the question if the proposed beta(1a) heptad repeat motif could be an active element of the DHPR-RyR1 signal transduction mechanism or already contributes at the ultrastructural level i.e. DHPR tetrad arrangement. Surprisingly, our experiments revealed full tetrad formation and an almost complete restoration of EC coupling in beta(1)-null zebrafish relaxed larvae and isolated myotubes upon expression of a beta(1a)-specific heptad repeat mutant (LVV to AAA) and thus contradict the earlier results.
2010 Elsevier Ltd. All rights reserved.
Figures
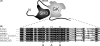

References
-
- Schneider M.F., Chandler W.K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation–contraction coupling. Nature. 1973;242:244–246. - PubMed
-
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation–contraction coupling in skeletal muscle. Nature. 1987;325:717–720. - PubMed
-
- Armstrong C.M., Bezanilla F.M., Horowicz P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N′-tetracetic acid. Biochim. Biophys. Acta. 1972;267:605–608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

