Evaluation of thieno[3,2-b]pyrrole[3,2-d]pyridazinones as activators of the tumor cell specific M2 isoform of pyruvate kinase
- PMID: 20451379
- PMCID: PMC2874658
- DOI: 10.1016/j.bmcl.2010.04.015
Evaluation of thieno[3,2-b]pyrrole[3,2-d]pyridazinones as activators of the tumor cell specific M2 isoform of pyruvate kinase
Abstract
Cancer cells have distinct metabolic needs that are different from normal cells and can be exploited for development of anti-cancer therapeutics. Activation of the tumor specific M2 form of pyruvate kinase (PKM2) is a potential strategy for returning cancer cells to a metabolic state characteristic of normal cells. Here, we describe activators of PKM2 based upon a substituted thieno[3,2-b]pyrrole[3,2-d]pyridazinone scaffold. The synthesis of these agents, structure-activity relationships, analysis of activity at related targets (PKM1, PKR and PKL) and examination of aqueous solubility are investigated. These agents represent the second reported chemotype for activation of PKM2.
Published by Elsevier Ltd.
Figures

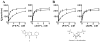
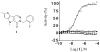
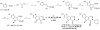

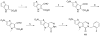
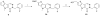
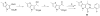
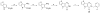
Similar articles
-
Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis.Nat Chem Biol. 2012 Oct;8(10):839-47. doi: 10.1038/nchembio.1060. Nat Chem Biol. 2012. PMID: 22922757 Free PMC article.
-
2-Oxo-N-aryl-1,2,3,4-tetrahydroquinoline-6-sulfonamides as activators of the tumor cell specific M2 isoform of pyruvate kinase.Bioorg Med Chem Lett. 2011 Nov 1;21(21):6322-7. doi: 10.1016/j.bmcl.2011.08.114. Epub 2011 Sep 14. Bioorg Med Chem Lett. 2011. PMID: 21958545 Free PMC article.
-
Small molecule activation of metabolic enzyme pyruvate kinase muscle isozyme 2, PKM2, circumvents photoreceptor apoptosis.Sci Rep. 2020 Feb 19;10(1):2990. doi: 10.1038/s41598-020-59999-w. Sci Rep. 2020. PMID: 32076076 Free PMC article.
-
A short review on cross-link between pyruvate kinase (PKM2) and Glioblastoma Multiforme.Metab Brain Dis. 2021 Jun;36(5):751-765. doi: 10.1007/s11011-021-00690-y. Epub 2021 Mar 2. Metab Brain Dis. 2021. PMID: 33651273 Review.
-
PKM2, cancer metabolism, and the road ahead.EMBO Rep. 2016 Dec;17(12):1721-1730. doi: 10.15252/embr.201643300. Epub 2016 Nov 17. EMBO Rep. 2016. PMID: 27856534 Free PMC article. Review.
Cited by
-
High-Resolution Molecular-Dynamics Simulations of the Pyruvate Kinase Muscle Isoform 1 and 2 (PKM1/2).Chemistry. 2025 Apr 4;31(20):e202402534. doi: 10.1002/chem.202402534. Epub 2025 Mar 15. Chemistry. 2025. PMID: 39614705 Free PMC article.
-
Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis.Nat Chem Biol. 2012 Oct;8(10):839-47. doi: 10.1038/nchembio.1060. Nat Chem Biol. 2012. PMID: 22922757 Free PMC article.
-
Regulation and function of pyruvate kinase M2 in cancer.Cancer Lett. 2013 Oct 10;339(2):153-8. doi: 10.1016/j.canlet.2013.06.008. Epub 2013 Jun 18. Cancer Lett. 2013. PMID: 23791887 Free PMC article. Review.
-
Anticancer agents that counteract tumor glycolysis.ChemMedChem. 2012 Aug;7(8):1318-50. doi: 10.1002/cmdc.201200176. Epub 2012 Jun 8. ChemMedChem. 2012. PMID: 22684868 Free PMC article. Review.
-
Cancer metabolic reprogramming: importance, main features, and potentials for precise targeted anti-cancer therapies.Cancer Biol Med. 2014 Mar;11(1):1-19. doi: 10.7497/j.issn.2095-3941.2014.01.001. Cancer Biol Med. 2014. PMID: 24738035 Free PMC article. Review.
References
-
- Devita VT Jr, Hellman S, Rosenberg SA, editors. Cancer Principles & Practice of Oncology. 7. Lippincott Williams & Wilkins; Philadelphia (PA): 2005.
-
- Warburg O. Science. 1956;123:309. - PubMed
-
- Warburg O. Science. 1956;124:269. - PubMed
-
- Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Semin Cancer Biol. 2005;15:300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

